MedCity News
MICHAEL SCHROEDER
May 3, 2022
With 1 in 8 women in the U.S. developing breast cancer in her lifetime and pathologists in short supply, there’s been a focus on improving technology to increase the chances of early detection and survival.
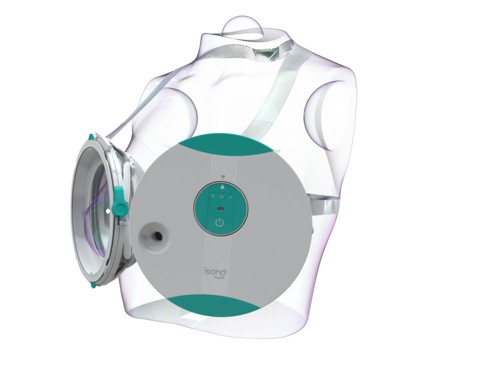
Now a company called iSono Health, which aims to make personalized whole breast imaging accessible to all women worldwide, has received FDA clearance for its automated, wearable, 3D breast ultrasound, the company announced Tuesday.
The technology, ATUSA, is designed to be efficient, accurate and comfortable, and will be accessible to patients and doctors at the point of care, iSono said.
In just two minutes, the portable system automatically scans the entire breast, independent of operator expertise, and offers 3D visualization of the breast tissue, according to the company.
The technology is integrated with machine learning models designed to provide clinical decision-making support.
In just two minutes, the portable system automatically scans the entire breast, independent of operator expertise, and offers 3D visualization of the breast tissue, according to the company.
The technology is integrated with machine learning models designed to provide clinical decision-making support.
iSono was founded in 2016 by two female engineers who shared similar personal experiences with the inadequacies of mammograms, especially for women with dense breasts, the company said.
“Mammograms have limited sensitivity in women who have dense breasts, which is about half of all women in the U.S.,” Maryam Ziaei, co-founder and CEO of the South San Francisco, California-based startup, said in an email. “Ultrasound has been proven effective in improving breast cancer detection in women who have dense breasts.”
iSono is continuing to study ATUSA to prove its effectiveness.
“We have demonstrated the safety and efficacy of the ATUSA device with extensive verification and validation testing as well as clinical feasibility studies for this FDA clearance,” Ziaei said. “However, we are conducting additional prospective studies.”
With the FDA’s clearance of ATUSA, iSono joins other companies seeking to improve cancer detection through the use of AI-powered technology.
In March, New York City-based Paige, a startup spun out of Memorial Sloan Kettering Cancer Center, launched software to help detect breast cancer that has spread to the lymph nodes.
Called Paige Breast Lymph Node, the AI-powered software aids pathologists- whose numbers have been declining-in the time-consuming task of finding small tumors.
Other companies like PathAI and Ibex also provide AI-powered decision support to help doctors make an accurate cancer diagnosis.
In March, New York City-based Paige, a startup spun out of Memorial Sloan Kettering Cancer Center, launched software to help detect breast cancer that has spread to the lymph nodes.
Other companies like PathAI and Ibex also provide AI-powered decision support to help doctors make an accurate cancer diagnosis.
“A portable and automated whole breast ultrasound augmented with machine learning would be the most practical technology to reduce breast cancer mortality globally, specifically in countries with limited resources,” said Dr. Mohammad Eghtedari, a breast radiologist and an assistant professor at University of California San Diego, in a statement accompanying iSono’s news release.
“A portable and automated whole breast ultrasound augmented with machine learning would be the most practical technology to reduce breast cancer mortality globally, specifically in countries with limited resources …
“In developed countries, ultrasound has been proven effective as an adjunct to mammography to improve breast cancer diagnosis in women with dense breasts.”
ATUSA would also provide another breast cancer screening option for younger women who are not eligible for mammograms, or women who otherwise lack of access to mammograms, Ziaei said.
The American Cancer Society recommends women of average risk for breast cancer begin being screened with mammography at age 45.
The U.S. Preventive Services Task Force recommends beginning breast cancer screening at age 50.
For women who are deemed to be at higher risk for breast cancer, and others who wish to start screening earlier, experts recommend first discussing potential benefits and harms of not only screening but subsequent treatment with a doctor.
Photo: iSono Health
Originally published at https://medcitynews.com on May 3, 2022.
Names mentioned
Maryam Ziaei, co-founder and CEO of the South San Francisco, California-based startup
Dr. Mohammad Eghtedari, a breast radiologist and an assistant professor at University of California San Diego