Scientific American
Mariana Lenharo
September 16, 2022
Site editor:
Joaquim Cardoso MSc.
health transformation — journal
September 16, 2022
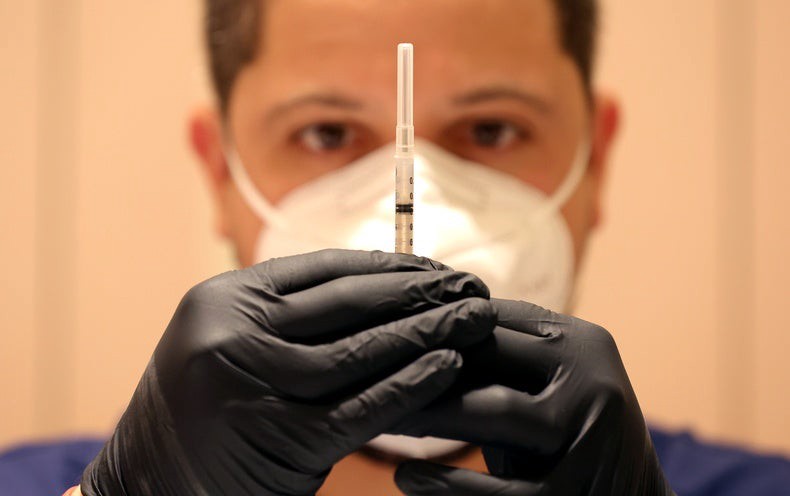
A new generation of COVID booster shots are now available to most people 12 years of age and older in the U.S.
The Food and Drug Administration authorized new formulations of the Moderna and Pfizer-BioNTech COVID vaccines for use as a single booster dose just a few weeks ago.
That decision was quickly endorsed by an immunization advisory panel at the U.S. Centers for Disease Control and Prevention.
The updated boosters target the newer, widely spread Omicron subvariants of the COVID-causing virus, BA.4 and BA.5, as well as the early 2020 form of the microbe, known as the ancestral strain.
On the week ending on September 10, BA.5 was estimated to be responsible for 87.5 percent of news infections in the U.S.
This is the first change for the Pfizer and Moderna shots since they were introduced two years ago, when they contained only genetic material from the ancestral form of the virus.
A new generation of COVID booster shots are now available to most people 12 years of age and older in the U.S.
The updated boosters target the newer, widely spread Omicron subvariants of the COVID-causing virus, BA.4 and BA.5, as well as the early 2020 form of the microbe, known as the ancestral strain.
The new booster shots are expected to trigger a better immune response against the new subvariants.
Clinical studies of similar two-part COVID boosters, as well as mouse research with these specific formulations, suggest that will be the case.
Still, some scientists point out we have no hard evidence the new shots will provide longer-lasting protection than previous boosters.
That’s an issue because people are tired of frequent requests to get yet another shot.
But the U.S. government is pushing for a rapid and wide rollout before an anticipated increase of cases in fall and winter months.
“It comes at a fortuitous time,” says epidemiologist Ali Khan, dean of the University of Nebraska Medical Center College of Public Health.
“As people congregate closer together in winter months, they’re more likely to spread microbes between them. So it’s great timing for what is expected to be a fall wave of cases.”
Clinical studies of similar two-part COVID boosters, as well as mouse research with these specific formulations, suggest that will be the case [for lasting protection]
Still, some scientists point out we have no hard evidence the new shots will provide longer-lasting protection than previous boosters.
Here is what we know about the makeup, effectiveness and safety of the new vaccine formulations, which are known as bivalent shots because they contain components of two versions of the virus.
We’ll also cover a few things that scientists are still trying to figure out.
Outline of the publication:
- INTRODUCTION
- WHAT ARE THE UPDATED BOOSTERS MADE OF?
- HOW EFFECTIVE ARE THE BOOSTERS?
- ARE THE NEW SHOTS SAFE?
- WHO IS ELIGIBLE?
- DO YOU NEED A BOOSTER IF YOU WERE RECENTLY INFECTED?
- ARE THERE OTHER LINGERING UNCERTAINTIES?
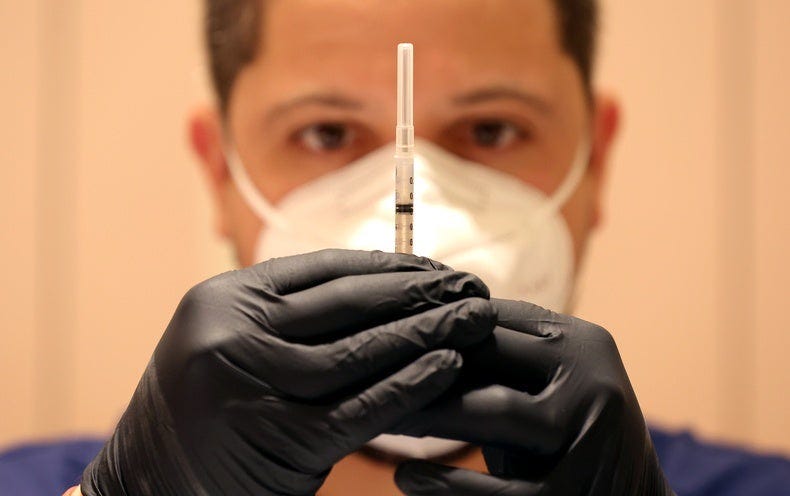
What are the updated boosters made of?
The Pfizer-BioNTech and Moderna COVID vaccines contain snippets of viral genetic material called mRNA.
Once injected, it can’t create a whole virus, but it does tell cells to build one isolated piece, the infamous spike protein found on the surface of SARS-CoV-2.
When the human immune system detects that protein, it starts to produce antibodies and activate other immune cells that can fight the actual virus.
The updated boosters contain mRNA instructions for both the ancestral spike protein and the one on Omicron BA.4 and BA.5. (The protein from those two subvariants is extremely similar.)
This is why the new shots induce a broader immune response.
All of the other ingredients-such as lipids, salts and acids, which help protect the mRNA and deliver it to our cells, balance acidity and maintain the stability of the vaccine-haven’t changed from the original formulation.
The overall dose of each updated booster is also the same as the original boosters.
“This is part of the benefit of having an mRNA vaccine, where you can change only the variants and keep everything else consistent,” says Gigi Gronvall, a senior scholar at the Johns Hopkins Center for Health Security.
“I wish that we had had these updated vaccines earlier.”

How effective are the boosters?
The FDA authorization was based on clinical trials of earlier versions of bivalent boosters (made with components of previous SARS-CoV-2 variants), mouse studies of the current bivalent boosters and real-world experience with the COVID mRNA vaccines.
Clinical trials of these newest formulations are still being conducted, so their data did not figure into the go-ahead decision.
But many scientists contend that the existing studies provide ample evidence of effectiveness.
In a clinical preprint study evaluating one of Moderna’s earlier bivalent booster versions-which was made with the ancestral form and the original Omicron variant, called BA.1-participants who received the booster had a 7.1-fold rise in neutralizing antibody levels against Omicron.
The ones who received the ancestral-only booster had a smaller, 3.8-fold rise in neutralizing antibody levels.
An advantage was also seen in neutralizing antibody levels against Omicron BA.4 and BA.5.
A similar result was achieved by an earlier bivalent booster developed by Pfizer and BioNTech.
It led to a 9.1-fold rise in neutralizing antibody levels against the original form of Omicron, compared with a 5.8-fold rise obtained by the ancestral-only booster.
A smaller advantage was seen in neutralizing antibody levels against BA.4 and BA.5.
The mouse studies of the new bivalent boosters showed they also increased neutralizing antibody levels, compared with earlier boosters.
This strategy of using previous clinical data and animal studies is what has been done with influenza vaccines for many years, notes immunologist Alessandro Sette of the La Jolla Institute for Immunology.
Flu shots are updated to target newer influenza strains without additional clinical studies.
When it comes to the new COVID shots, “obviously, if more data was available, specifically immunogenicity data in humans, that would be better,” Sette says.
“But I still think it’s a reasonable path to take.” Like Gronvall, his confidence is buoyed because the overall structure of the updated boosters is very similar to the original ones.
This strategy of using previous clinical data and animal studies is what has been done with influenza vaccines for many years, notes immunologist Alessandro Sette of the La Jolla Institute for Immunology.
Flu shots are updated to target newer influenza strains without additional clinical studies.
Though Sette is reassured, some other experts believe that the lack of human data about these particular shots could turn into a problem when it comes to building public trust.
Epidemiologist Michael Osterholm, director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, says he worries that if people have a concern about the safety or efficacy of the updated boosters, they might not get the shots.”
I was hoping we would have immunogenicity data in humans to be able to say that, actually, the immune response is better or at least similar to the current vaccine,” he adds.

Are the new shots safe?
Osterholm himself is confident about safety. “I do not believe that we need any additional safety data.
We have no reason to believe that there’s going to be any difference in safety with regards to the current vaccines versus the ones we’ve been using,” he says.
Clinical trials of the earlier bivalent booster versions show that they have a risk profile very much like the original vaccines and boosters.
The clinical study that evaluated the earlier version of Moderna’s bivalent booster showed that the most commonly reported side effects were typical, including pain, redness and swelling at the injection site, fatigue, and some nausea and fever.
An early version of a bivalent shot from Pfizer and BioNTech had a similar profile.
Overall, the mRNA vaccines have been administered to millions of people around the world, says epidemiologist William Moss, executive director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.
“We may have more information on these vaccines than almost any other vaccine,” he says.
“I’m comfortable that this is sufficient evidence to make a policy decision like this.”

Who is eligible?
First, you must be fully vaccinated.
For most people, that means you have received two primary doses of vaccines made by Moderna, Novavax, or Pfizer and BioNTech or one primary dose of the shot made by Johnson & Johnson’s subsidiary Janssen.
At least two months must have passed since you completed this primary vaccination series or received your most recent booster.
First, you must be fully vaccinated.
At least two months must have passed since you completed this primary vaccination series or received your most recent booster.
Under those conditions, people age 18 and older are eligible for the new booster from Moderna, and people age 12 and older are eligible for the one from Pfizer and BioNTech.
The situation is slightly different if you are immunocompromised -for instance, if you have a weakened immune system because of a current cancer treatment or an organ transplant.
In that case, you must have received three primary doses of Moderna or Pfizer-BioNTech, two primary doses of Novavax or one primary dose of Janssen plus an additional dose of Pfizer-BioNTech or Moderna. No booster is required to be considered fully vaccinated.
Although children under age 12 are not eligible yet, this could change in the upcoming months.
Pfizer and BioNTech expect to file an FDA submission for children ages five to 11 sometime in October, the companies said in late August.
There are a few reasons that could explain the delay, Khan notes.
“We know, for example, that children require different doses than adults,” he says, adding that there is also more scrutiny and rigor around authorizing vaccination for kids.
People who are not sure if they are up-to-date with their vaccines can use a CDC tool to determine their eligibility for a booster (just click on the button “Find Out When to Get a Booster” and answer a few questions to get a personalized recommendation). You can search for locations offering the updated boosters here.

Do you need a booster if you were recently infected?
Moss notes that the status of an individual’s COVID immunity results from a combination of factors, including the number of vaccine doses they received, if they were infected with the virus and the timing of all those events.
“We can’t test everyone to see where they are in terms of their antibodies against BA.5 and decide who needs and who does not need vaccination based on that,” Moss says.
“I would say that if someone knows they were infected with BA.5-and that’s a lot of people-they should probably still get the booster.”
He notes that although people who have been infected with Omicron may retain some natural immunity to the virus, they will probably get additional protection from the booster.
Such people should consider waiting three months postinfection before getting the new shot, according to CDC recommendations, and people with a current COVID infection should wait until they are fully recovered.
Highly vulnerable groups, such as older adults or the immunocompromised, will likely benefit the most, Moss adds.
Highly vulnerable groups, such as older adults or the immunocompromised, will likely benefit the most, Moss adds.

Are there other lingering uncertainties?
The ancestral version of the virus that causes COVID, SARS-CoV-2, is no longer circulating, crowded out by more successful variants such as Delta and Omicron.
So some experts wonder why it is still in the recipe of the updated boosters.
That question was raised in a recent preprint paper that hasn’t been peer-reviewed yet.
The article was written by immunologist Rafi Ahmed, director of the Emory University Vaccine Center, and his colleagues.
“It is highly unlikely that a new variant will emerge from the [ancestral] virus. From that perspective, boosting with [it] doesn’t make much sense,” Ahmed says.”
The other issue, which I’m surprised that not many people are discussing, is that when you combine the two, you reduce the dose of Omicron.”
The original Moderna booster, for example, had a 50-microgram dose. The updated booster contains 25 micrograms of the original-virus-based vaccine and 25 micrograms of the Omicron BA.4/BA.5 vaccine.
“So you’re reducing the vaccine dose, which is always a concern that it could be less effective,” Ahmed says.
Another uncertainty about the updated boosters is how long their protection will last.
Achieving durable immunity from vaccines is one of the biggest challenges at the moment, Osterholm notes.
Studies suggest that the protection conferred by the original mRNA booster against Omicron wanes after a few months, which is why we’ve needed repeated boosters.
Studies suggest that the protection conferred by the original mRNA booster against Omicron wanes after a few months, which is why we’ve needed repeated boosters.
His group at CIDRAP is leading an effort to create a roadmap for COVID vaccine development, which includes efforts to make the immunity from shots last longer.
“I think one day the vaccines that we have now will be seen as having been very important,” he says. “But they’ll be replaced, hopefully, by vaccines that can give us more durable protection over time and [against] a wider range of variants.”
Originally published at https://www.scientificamerican.com.
About the author:
Mariana Lenharo is a science and health journalist with a Masters in journalism from Columbia University
Names mentioned:
epidemiologist Ali Khan, dean of the University of Nebraska Medical Center College of Public Health
Gigi Gronvall, a senior scholar at the Johns Hopkins Center for Health Security.
Alessandro Sette of the La Jolla Institute for Immunology.
Epidemiologist Michael Osterholm, director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota
epidemiologist William Moss, executive director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.
immunologist Rafi Ahmed, director of the Emory University Vaccine Center, and his colleagues.