Health & Care Transformation
Management Institute
Joaquim Cardoso MSc*
Chief Strategy Officer, Research and Editor
November 9, 2022
*MSc from London Business School — MIT Sloan Masters Program
Source: Koen Kas
Linkedin
Koen Kas
November, 2022
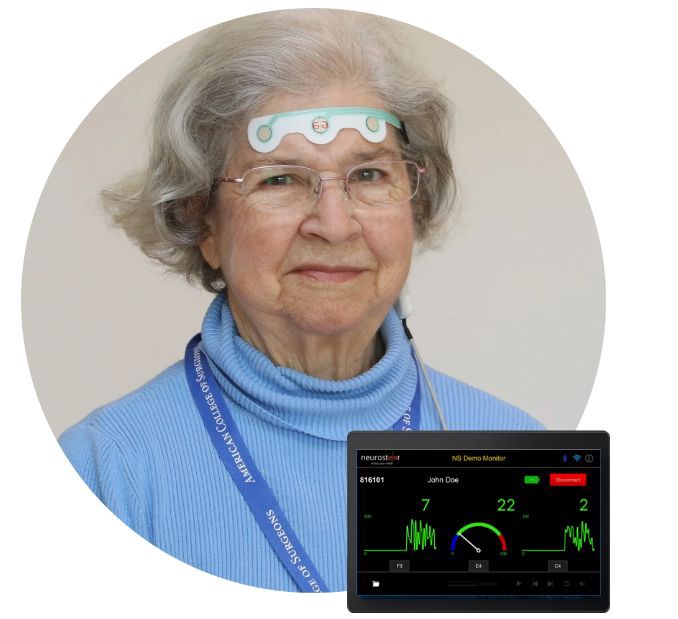
A portable brain monitor
The FDA has granted 510(k) clearance to Neurosteer’s electroencephalogram (EEG) device, a portable, pocket-sized brain monitoring platform that uploads neural data to the cloud for clinical assessment.
The system includes a dry gel adhesive electrode strip connected to a sensor as well as a tablet-like brain activity monitor and cloud-based software.
The forehead electrode strip avoids the interference that can come from hair and can remain in place for up to 12 hours.
The system includes a dry gel adhesive electrode strip connected to a sensor as well as a tablet-like brain activity monitor and cloud-based software.
The forehead electrode strip avoids the interference that can come from hair and can remain in place for up to 12 hours.
The system combines traditional EEG frequency bands with several novel brain metric visual representations and auditory prompts to help clinicians in early detection of brain deterioration.
The clearance allows the system to be used in a range of clinical settings, such as intensive care units, physicians’ offices and drug trials, with an initial focus on the early detection of cognitive decline and other neurological disorders.
The clearance allows the system to be used in a range of clinical settings, such as intensive care units, physicians’ offices and drug trials, with an initial focus on the early detection of cognitive decline and other neurological disorders.