Institute4HealthTransformation (i4ht)
Joaquim Cardoso MSc
Founder and Chief Researcher & Editor
December 18,2022
EXECUTIVE SUMMARY
Background
- Whether ivermectin, with a maximum targeted dose of 600 μg/kg, shortens symptom duration or prevents hospitalization among outpatients with mild to moderate coronavirus disease 2019 (COVID-19) remains unknown.
- Our objective was to evaluate the effectiveness of ivermectin, dosed at 600 μg/kg, daily for 6 days compared with placebo for the treatment of early mild to moderate COVID-19.
Methods
- ACTIV-6, an ongoing, decentralized, randomized, double-blind, placebo-controlled, platform trial, was designed to evaluate repurposed therapies in outpatients with mild to moderate COVID-19.
- A total of 1206 participants age ≥30 years with confirmed COVID-19, experiencing ≥2 symptoms of acute infection for ≤7 days, were enrolled from February 16, 2022, through July 22, 2022, with follow-up data through November 10, 2022, at 93 sites in the US.
- Participants were randomized to ivermectin, with a maximum targeted dose of 600 μg/kg (n=602), daily vs. placebo daily (n=604) for 6 days. The primary outcome was time to sustained recovery, defined as at least 3 consecutive days without symptoms.
- The 7 secondary outcomes included a composite of hospitalization, death, or urgent/emergent care utilization by day 28.
Results
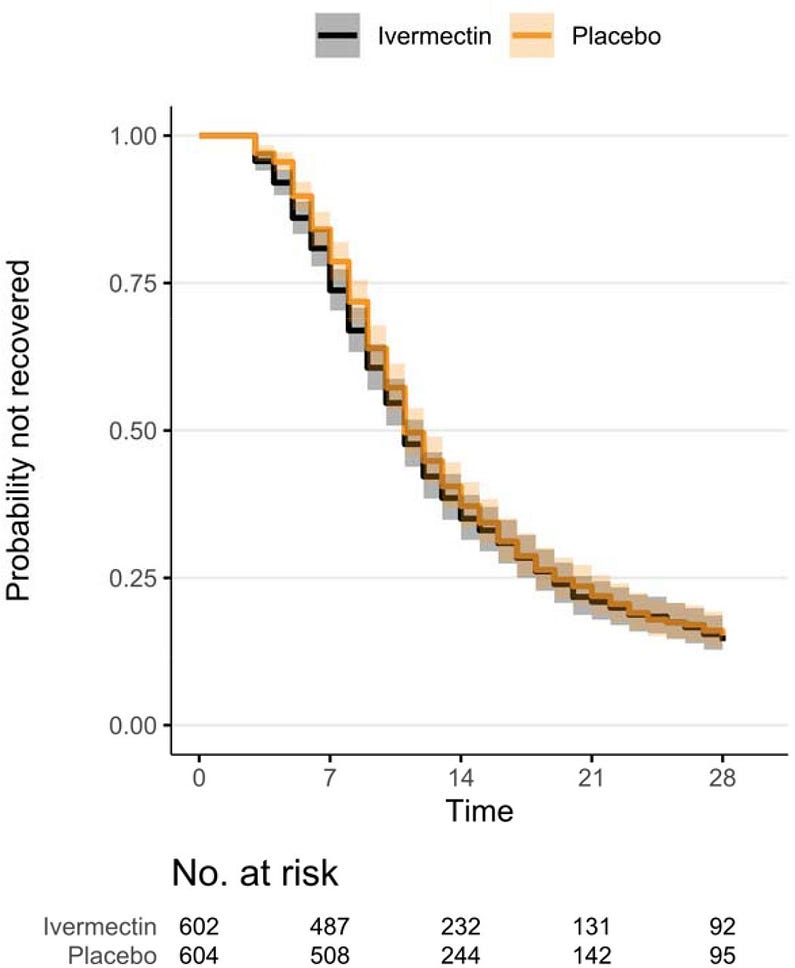
- Among 1206 randomized participants who received study medication or placebo, median (interquartile range) age was 48 (38–58) years; 713 (59%) were women; and 1008 (84%) reported ≥2 SARS-CoV-2 vaccine doses. Median time to recovery was 11 (11–12) days in the ivermectin group and 11 (11–12) days in the placebo group.
- The hazard ratio (HR) (95% credible interval [CrI], posterior probability of benefit) for improvement in time to recovery was 1.02 (0.92–1.13; P[HR>1]=0.68).
- In those receiving ivermectin, 34 (5.7%) were hospitalized, died, or had urgent or emergency care visits compared with 36 (6.0%) receiving placebo (HR 1.0, 0.6– 1.5; P[HR<1]=0.53).
- In the ivermectin group, 1 participant died and 4 were hospitalized (0.8%); 2 participants (0.3%) were hospitalized in the placebo group and there were no deaths.
- Adverse events were uncommon in both groups.
Conclusions
- Among outpatients with mild to moderate COVID-19, treatment with ivermectin, with a maximum targeted dose of 600 μg/kg daily for 6 days, compared with placebo did not improve time to recovery.
- These findings do not support the use of ivermectin in patients with mild to moderate COVID-19.
Infographic
Figure 3. — Kaplan-Meier for Primary Outcome of Time to Sustained Recovery.
ORIGINAL PUBLICATION
Effect of Ivermectin 600 μg/kg for 6 days vs Placebo on Time to Sustained Recovery in Outpatients with Mild to Moderate COVID-19: A Randomized Clinical Trial
MedRxiv
Susanna Naggie, David R. Boulware, Christopher J. Lindsell, Thomas G. Stewart, Stephen C. Lim, Jonathan Cohen, David Kavtaradze, Arch P. Amon, Ahab Gabriel, Nina Gentile, G. Michael Felker, Russell L. Rothman, Dushyantha Jayaweera, Matthew W. McCarthy, Mark Sulkowski, Sybil Wilson, Allison DeLong, April Remaly, Rhonda Wilder, Sean Collins, Sarah E. Dunsmore, Stacey J. Adam, Florence Thicklin, George J. Hanna, Adit A. Ginde, Mario Castro, Kathleen McTigue, Elizabeth Shenkman, Adrian F. Hernandez
December 15, 2022
Introduction
Despite treatment advances for coronavirus disease 2019 (COVID-19), the evolution of SARSCoV-2 variants and subvariants has shifted therapeutic options with loss of effectiveness of monoclonal antibodies.
Novel oral antivirals have been authorized for high-risk individuals in high-income countries.1,2
However, efficacy of these antivirals in those vaccinated or with prior infection remains unclear.
Interest remains for the potential of repurposed drugs to improve symptoms and clinical outcomes in patients with COVID-19.
Numerous repurposed drugs have been investigated for COVID-19, with several large, randomized outpatient trials published.3–5 Trial results have been mixed.
Some suggest promise to reduce emergency department (ED) visits or hospitalizations for drugs including fluvoxamine at 100 mg twice daily3 and immediate-release metformin.6
Others have failed to show a reduction in emergency department visits or hospitalizations such as fluvoxamine 50 mg twice daily.6
While recently completed trials benefit from increasing representation of vaccinated people, which is more relevant to the pandemic’s current state, the results have not impacted treatment guidelines due to study design and regulatory requirements.7–9
Ivermectin, an anti-parasitic drug used worldwide for onchocerciasis and strongyloidiasis, emerged in 2020 as a potential repurposed drug for COVID-19 informed by an in vitro study suggesting possible anti-viral activity.10
The interest for ivermectin as a COVID-19 therapeutic has remained high and, while there have been numerous ivermectin studies, its use has become controversial due to a lack of high-quality, adequately powered randomized trials and article retractions of some of the earlier and most positive studies.11–14
Three large randomized outpatient trials in people with symptomatic mild or moderate COVID-19 failed to identify a clinical benefit of ivermectin when dosed at 400 µg/kg daily for 3 days.15–17
One possibility is that the dose and duration studied was too low and too short, missing the therapeutic window for ivermectin.
A proof-of-concept randomized controlled clinical trial in hospitalized patients showed that ivermectin, 600 µg/kg, for 5 days suggested possible anti-viral activity with increasing dose.17,18
For this reason we tested ivermectin, with a maximum targeted dose of 600 µg/kg, daily for 6 days on the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) platform from February 16, 2022 through July 22, 2022.
Three large randomized outpatient trials in people with symptomatic mild or moderate COVID-19 failed to identify a clinical benefit of ivermectin when dosed at 400 µg/kg daily for 3 days.15–17
For this reason we tested ivermectin, with a maximum targeted dose of 600 µg/kg, daily for 6 days on the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) platform from February 16, 2022 through July 22, 2022.
ACTIV-6 is an ongoing, fully remote (decentralized), double-blind, randomized, placebocontrolled, platform trial investigating repurposed drugs for the treatment of mild to moderate COVID-19 in the outpatient setting.
This report describes the effectiveness of this dose and duration of ivermectin compared with blinded placebo for the treatment of early mild to moderate COVID-19.

METHODS & OTHER SECTIONS
See the original publication (this is an excerpt version)
DISCUSSION
Among a largely vaccinated outpatient population with COVID-19, treatment with ivermectin, with a targeted maximum dose of 600 µg/kg, daily for 6 days compared with placebo was not shown to improve time to recovery in over 1200 participants in the US during omicron variant/subvariant circulation.
No evidence of benefit was observed for secondary clinical outcomes including the composite of hospitalization, death, or acute care visits. Hospitalization and death were uncommon in this largely vaccinated population. These find
Multiple large, double-blind randomized controlled trials have failed to identify a clinically meaningful benefit of ivermectin when used at a targeted dose of 400 µg/kg daily for 3 days.6,16
This large clinical trial addresses a potential gap in knowledge by testing 1) a higher daily dose (targeted maximum dose of 600 µg/kg) and 2) longer 6-day duration of ivermectin,. Due to the lack of early phase studies or animal model studies to determine optimal dosing for a therapeutic drug, the appropriate dosing of ivermectin for COVID-19 was never determined. A combination of modeling studies and a proof-of-concept clinical study have suggested doses up to 600 µg/kg daily may achieve system levels sufficient for in vitro antiviral activity.17,18 Although a phase 2 trial testing ivermectin 600 µg/kg daily for 7 days and assessing a virologic endpoint of oropharyngeal SARS-CoV-2 PCR did not show measurable antiviral activity and was stopped for futility.21 This dose was safe and generally well tolerated, with a higher prevalence of the known self-resolving visual disturbances in the intervention arm previously reported with similar doses of ivermectin for parasitic infections.17,18
Recent results from the current platform trial reported participants receiving ivermectin, with a 400 µg/kg, daily for 3 days had a an average ~12-hour shorter time spent unwell when compared with placebo, a secondary outcome for the trial.15
This finding was not replicated here. The notable difference in baseline characteristics between these 2 cohorts is the completed vaccination rate, which is 84% for this study and was 47% for the prior ivermectin 400 µg/kg group.15 Hospitalizations and COVID-19-related clinical events were less common in this largely vaccinated cohort. The incidence of acute care visits, hospitalizations, or death was similar with ivermectin (5.7%) and placebo (6.0%); a result also observed in the two prior ivermectin 400 μg/kg randomized trials in the US.6,15
This trial has several strengths.
This was a double-blind, randomized, placebo-controlled nationwide trial with 93 enrolling sites and a call center that recruited participants from all 50 US states. The ivermectin 600 µg/kg group of the platform trial enrolled rapidly due to ongoing omicron variant/subvariant surges and largely included vaccinated people, thus representing a highly relevant study population that also addresses a weakness of many other studies that excluded vaccinated people.

Limitations
This study has limitations.
Due to infrequent hospitalization, we cannot draw definitive inferences on whether ivermectin impacts hospitalization without much larger trials.
Also, due to the remote nature of the trial, 60% of participants received study drug within 5 days of symptom onset.
Most outpatient COVID-19 antiviral trials have limited enrollment to participants within 5 days of symptom onset.1,2
In this trial, we observed no evidence of a differential treatment effect based on shorter time to study drug receipt.

Conclusions
Among outpatients with mild or moderate COVID-19, treatment with ivermectin, with a targeted maximum dose of 600 μg/kg daily for 6 days, was not shown to improve time to recovery compared with placebo.
These findings do not support the use of ivermectin in outpatients with COVID-19.
Among outpatients with mild or moderate COVID-19, treatment with ivermectin, with a targeted maximum dose of 600 μg/kg daily for 6 days, was not shown to improve time to recovery compared with placebo.
These findings do not support the use of ivermectin in outpatients with COVID-19.
Originally published at https://www.medrxiv.org on December 15, 2022.
Related Publications
Why You Shouldn’t Use Ivermectin to Treat or Prevent COVID-19 (FDA)
Por que você não deve usar Ivermectina para tratar ou prevenir a COVID
Durante a pandemia da COVID-19, alguns consumidores parecem estar cada vez mais interessados em recorrer ao uso da…www.fda.gov





