transforming health
institute for continuous health transformation
& digital health
Joaquim Cardoso MSc
Founder and Chief Researcher & Editor
December 25, 2022
Key Message:
- The antiviral Molnupiravir (Lageviro) is widely used across the world to treat SARS-CoV-2 infection.
- This study demonstrates that this commonly used antiviral can supercharge viral evolution in immunocompromised patients, potentially generating new variants and prolonging the pandemic.
MedRxiv
Nicholas M. Fountain-Jones1,2*, Robert Vanhaeften1 , Jan Williamson1 3 ,
Janelle Maskell1 , I-Ly J Chua1 , Michael Charleston 2 & Louise Cooley 1,3 4 5
December 22, 2022
Abstract
- The antiviral Molnupiravir (Lageviro) is widely used across the world to treat SARS-CoV-2 infection.
- Molnupiravir reduces viral replication by inducing mutations throughout the genome, yet in patients that do not clear the infection, the longer-term impact of the drug on virus evolution is unclear.
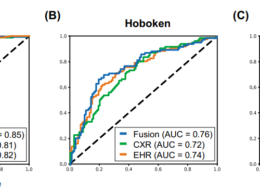
- Here, we used a case-control approach to monitor SARS-CoV-2 genomes through time in nine immunocompromised -patients with five treated with Molnupiravir.
- Within days of treatment, we detected a large number of low-frequency mutations in patients and that these new mutations could persist and, in some cases, were fixed in the virus population.
- All patients treated with the drug accrued new mutations in the spike protein of the virus, including non-synonymous mutations that altered the amino acid sequence.
- Our study demonstrates that this commonly used antiviral can supercharge viral evolution in immunocompromised patients, potentially generating new variants and prolonging the pandemic.
Our study demonstrates that this commonly used antiviral can supercharge viral evolution in immunocompromised patients, potentially generating new variants and prolonging the pandemic.

Funding Statement
This project was supported by an Australian Research Council Discovery Project Grant (DP190102020).
Data Availability
All data will be available online at GISAID. The sequence alignment is available upon request.
Originally published at https://www.medrxiv.org on December 22, 2022.
About the authors & affiliations
Nicholas M. Fountain-Jones1,2*,
Robert Vanhaeften1 ,
Jan Williamson1 3 ,
Janelle Maskell1 ,
I-Ly J Chua1 ,
Michael Charleston 2 &
Louise Cooley1,3 4 5
1 Royal Hobart Hospital,
Pathology Department, Hobart Australia 7001.
2 School of Natural Sciences,
University of Tasmania, Hobart Australia 7001.
3 School of Medicine,