IHME – Institute for Health Metrics and Evaluation
May 21, 2021
To project future COVID-19 trends, IHME centralizes and updates all available data on vaccine efficacy. This document summarizes the available data and key underlying assumptions of IHME’s projections. This page is updated monthly; for interim updates please see the “Methods updates” section of our weekly policy briefings.
REVIEW AND SUMMARY OF AVAILABLE DATA
Using all publications, reports, and news articles, we review all available data to find how effective vaccines are at achieving multiple outcomes. “Vaccine efficacy” is not a single number – we capture:
- Prevention of symptomatic disease: a vaccine’s efficacy at causing an exposed individual not to suffer the symptoms of COVID-19 infection. The person can contract the virus but will not develop disease.
- Prevention of severe disease: a vaccine’s efficacy at preventing an exposed person from developing more serious symptoms that often require hospitalization.
- Prevention of infection: a vaccine’s efficacy at stopping transmission of the virus from one person to another. An exposed person will not contract the virus, and by definition they will also not develop symptoms or disease.
For each of the outcomes above, we also capture the differences by:
- First dose versus complete regimen (not applicable for 1-dose vaccines, like Johnson & Johnson)
- Asymptomatic, any symptomatic, or severe disease
- Variant of COVID-19: D614G (ancestral type*), B.1.1.7, B.1.351, P.1, B.1.617
*Ancestral type is the predominant global strain of a virus.
Where genomic sequencing of cases did not occur, we use the location of the study as a proxy for the predominant variant type. For example, studies in South Africa were assumed to represent efficacy against B.1.351.
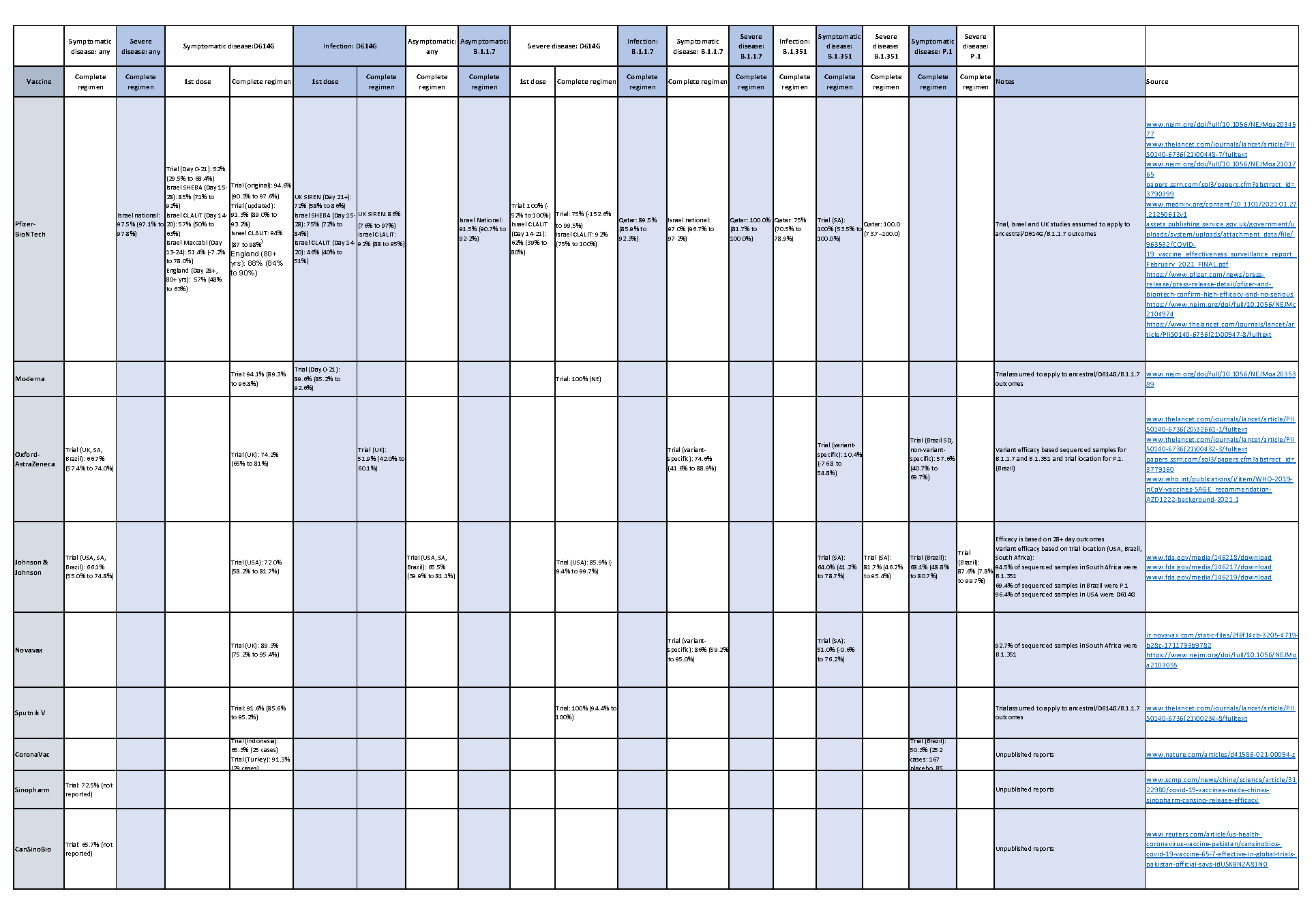
Data available today consist of clinical trials and several quasi-observational studies. Table 1 summarizes the available data to date:
Table 1: Available vaccine efficacy data (last updated May 14, 2021)
ESTIMATING VACCINE EFFICACY FOR COVID-19 PROJECTIONS
Currently, the IHME model uses the following inputs of vaccine efficacy, separated by variant:
- Efficacy at preventing symptomatic disease
- Efficacy at preventing infection
Importantly, vaccine outcomes do not seem to differ for D614G and B.1.1.7. We assume the efficacy against B.1.351 is the same for P.1, as there are currently very limited data for the P.1 variant, and these two variants have a similar mutation. Given the absence of any specific data, we also assume the efficacy for B.1.617 is the same as B.1.351 and P.1.
Wherever data are available (see Table 1) we use available data, and where we do not yet have data, we take different approaches based on variant, vaccine, and outcome. We make estimates to complete the empty cells in Table 2:
Table 2: Vaccine efficacy by coronavirus variant, available data
| Vaccine | Efficacy at preventing disease:
D614G & B.1.1.7 |
Efficacy at preventing infection:
D614G & B.1.1.7 |
Efficacy at preventing disease:
B.1.351, P.1, B.1.617 |
Efficacy at preventing infection:
B.1.351 P.1 |
| Pfizer/BioNTech | 91% | |||
| Moderna | 94% | |||
| AstraZeneca | 74% | 52% | 10% | |
| Johnson & Johnson (Janssen) | 72% | 72% | 64% | |
| Sputnik-V | 92% | |||
| Novavax | 89% | 49% | ||
| CoronaVac | 50% | |||
| Sinopharm | 73% | |||
| Tianjin CanSino | 66% | |||
| Covaxin | 78% | |||
| Other mRNA vaccines | ||||
| All other vaccines |
Table 3 shows the final model inputs for vaccine efficacy at preventing disease and infection, by vaccine and variant type.
Table 3: Vaccine efficacy by coronavirus variant, available data, and modeled estimates
| Vaccine | Efficacy at preventing disease:
D614G & B.1.1.7 |
Efficacy at preventing infection:
D614G & B.1.1.7 |
Efficacy at preventing disease:
B.1.351, P.1, B.1.617 |
Efficacy at preventing infection:
B.1.351, P.1, B.1.617 |
| Pfizer/BioNTech | 91% | 86% | 76% | 72% |
| Moderna | 94% | 89% | 79% | 75% |
| AstraZeneca | 74% | 52% | 10% | 9% |
| Johnson & Johnson (Janssen) | 72% | 72% | 64% | 56% |
| Sputnik-V | 92% | 81% | 70% | 61% |
| Novavax | 89% | 79% | 49% | 43% |
| CoronaVac | 50% | 44% | 38% | 33% |
| Sinopharm | 73% | 65% | 55% | 49% |
| Tianjin CanSino | 66% | 58% | 50% | 44% |
| Covaxin | 78% | 69% | 59% | 52% |
| Other mRNA vaccines | 91% | 86% | 76% | 72% |
| All other vaccines | 75% | 66% | 57% | 50% |
_______________________________________________
NOTES
| To estimate efficacy at preventing disease for D614G and B.1.1.7 |
mRNA vaccines: We assume the same efficacy as Pfizer-BioNTech (9%).
CoronaVac: We use the Brazil arm of the CoronaVac trial, as it has the largest number of cases.
All other vaccines: We assume 75% efficacy.
| To estimate efficacy at preventing infection for D614G and B.1.1.7 |
Moderna: We use the ratio between preventing asymptomatic infection over all cases (.95) from the Pfizer-BioNTech trial.
Johnson & Johnson: Based on trial results, we assume the effect is the same for preventing disease and infection.
All other vaccines: We use the average infection-to-disease ratio from Pfizer-BioNTech, Johnson & Johnson, and Oxford-AstraZeneca .
| To estimate efficacy and preventing disease for variants B.1.351, P.1, and B.1.617 |
mRNA Vaccines: We find the ratio of the efficacy between B.1.351 and B.1.1.7 as observed in the Qatar study.
All other vaccines without available data: We apply the average B.1.351/P.1:D614G/B.1.1.7 ratio for Novavax and Johnson & Johnson to determine the vaccine-specific efficacy at preventing disease.
| To estimate efficacy at preventing infection for variants B.1.351, P.1, and B.1.617 |
All vaccines: Use same infection-to-disease ratio between D614G and B.1.351, and apply this ratio to estimate efficacy at preventing infection.
First published at http://www.healthdata.org
http://www.healthdata.org/covid/covid-19-vaccine-efficacy-summary