the health strategist
research and strategy institute — for continuous transformation
in value based health, care & tech
Joaquim Cardoso MSc.
Chief Researcher & Editor of the site
March 29, 2023
EXECUTIVE SUMMARY
A study was conducted to assess whether there is an increased risk of cardiovascular events, such as stroke, myocardial infarction, and pulmonary embolism, …
… after the administration of a bivalent messenger RNA vaccine targeting both the ancestral and omicron BA.4–BA.5 sublineages of SARS-CoV-2.
- The study used comprehensive data from the French National Health Data System linked to the national Covid-19 vaccination database.
- The study included all persons who were 50 years of age or older and had received a booster dose between October 6 and November 9, 2022, when both monovalent and bivalent vaccines were being administered in France.
The study found that there was no evidence of an increased risk of cardiovascular events among the recipients of the bivalent vaccine as compared with recipients of the monovalent vaccine.
- The results provide reassurance regarding the continued use of this bivalent vaccine.
DEEP DIVE

Stroke, Myocardial Infarction, and Pulmonary Embolism after Bivalent Booster
NEJM
Marie-Joelle Jabagi, Pharm.D., Ph.D.
Marion Bertrand, M.Sc.
Jérémie Botton, Pharm.D., Ph.D.
Stéphane Le Vu, Pharm.D., Ph.D.
Alain Weill, M.D.
Rosemary Dray-Spira, M.D., Ph.D.
Mahmoud Zureik, M.D., Ph.D.
EPI-PHARE Scientific Interest Group, Saint-Denis, France
To the Editor
A bivalent messenger RNA vaccine targeting both the ancestral and omicron BA.4–BA.5 sublineages of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Pfizer–BioNTech) was introduced in France in early October 2022 and recommended for booster vaccination in vulnerable populations.
Between October 6 and November 9, both monovalent and bivalent vaccines were available for administration to persons who were 50 years of age or older.
In January 2023, the Vaccine Safety Datalink of the Centers for Disease Control and Prevention alerted the public about a possible increased risk of ischemic stroke within the 21 days after the bivalent injection in persons 65 years of age or older.1
We previously found no increase in the incidence of stroke, acute myocardial infarction, or pulmonary embolism after administration of the monovalent vaccine.2,3
Thus, we wanted to assess whether the risk of such events differed after receipt of the bivalent booster as compared with the monovalent booster.
In this population-based study, we used comprehensive data from the French National Health Data System linked to the national coronavirus disease 2019 (Covid-19) vaccination database.
All persons who were 50 years of age or older and who had received a booster dose between October 6 and November 9, 2022, were included in the study.
This time window captured the only period in which both vaccines were being administered in France.
During this period, the uptake of the bivalent vaccine overtook the uptake of the monovalent vaccine, with 932,583 persons receiving the bivalent vaccine and 121,362 receiving the monovalent vaccine (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
For each day during the study period, we matched each recipient of the monovalent vaccine with up to five randomly sampled recipients of the bivalent vaccine on the same day (Fig. S2).
Recipients were followed until 21 days after vaccination.
We estimated the risks of ischemic stroke, hemorrhagic stroke, myocardial infarction, and pulmonary embolism associated with the bivalent vaccine as compared with the monovalent vaccine by calculating hazard ratios as determined by propensity score–weighted Cox models (see the Supplementary Methods).4,5
Of a total of 470,962 vaccine recipients (mean [±SD] age, 72.6±10.4 years), 97,234 (20.6%) received the monovalent vaccine and 373,728 (79.4%) received the bivalent vaccine (Fig. S2 and Table S1).
After inverse probability of treatment weighting, sociodemographic and health-status characteristics were well balanced between the two groups (Fig. S3).
At 21 days after the booster dose, we found no evidence of an increased risk of cardiovascular events among the recipients of the bivalent vaccine as compared with recipients of the monovalent vaccine.
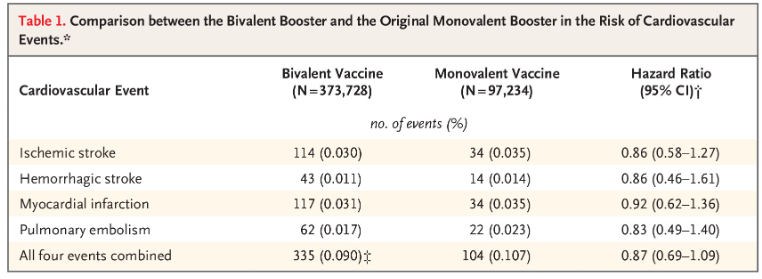
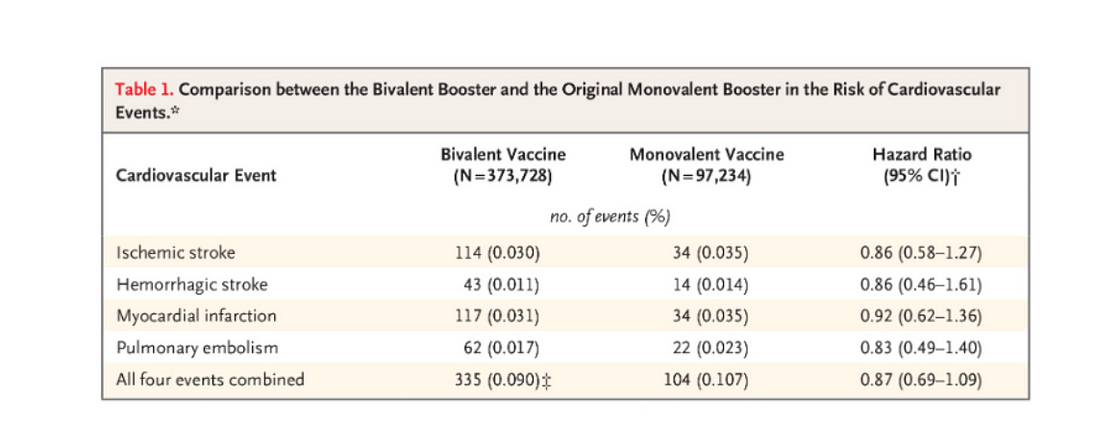
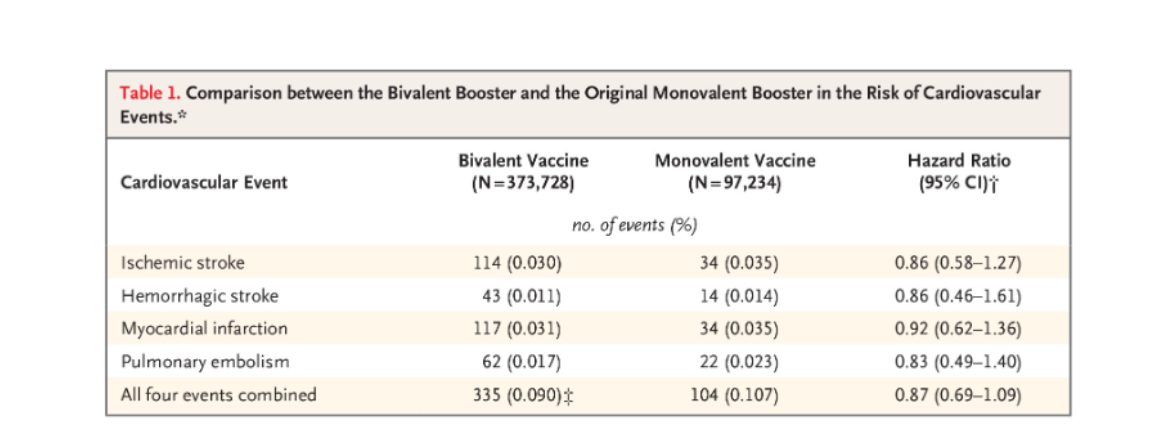
The evaluated events included ischemic stroke (hazard ratio, 0.86; 95% confidence interval [CI], 0.58 to 1.27), hemorrhagic stroke (hazard ratio, 0.86; 95% CI, 0.46 to 1.61), myocardial infarction (hazard ratio, 0.92; 95% CI, 0.62 to 1.36), pulmonary embolism (hazard ratio, 0.83; 95% CI, 0.49 to 1.40), and all four events combined (hazard ratio, 0.87; 95% CI, 0.69 to 1.09) (Table 1).
Thus, our results provide reassurance regarding the continued use of this bivalent vaccine.