the health strategist
institute for strategic health transformation
& digital health
Joaquim Cardoso MSc.
Chief Research and Strategy Officer (CRSO),
Chief Editor and Senior Advisor
September 19, 2023
What is the message?
Centers for Medicare and Medicaid Services (CMS) is preparing to negotiate prices for certain high-cost drugs with pharmaceutical manufacturers, aiming to reduce prescription drug costs for Medicare beneficiaries.
This negotiation process, established as a result of the Inflation Reduction Act signed by President Biden in 2022, marks the first time the federal government will negotiate drug prices on behalf of Medicare beneficiaries, beginning in 2026.
The article discusses concerns raised by drug manufacturers and stakeholders who believe the CMS process could lead to government price setting.
However, the authors argue that CMS is creating a fair negotiation process that encourages trust and good-faith negotiations.
The article outlines the CMS negotiation process, emphasizing confidentiality during negotiations and plans to publish an explanation of the negotiations for transparency.
DEEP DIVE
CMS Set to Negotiate with Pharmaceutical Manufacturers on Drug Prices
The Common Wealth Fund
Kate Meyer and Jeremy Sharp
September 8, 2o23

President Joe Biden speaks during an event celebrating the lowering of drug pricing in the East Room of the White House on August 29, 2023 in Washington D.C. Photo: Nathan Posner/Anadolu Agency via Getty Images
TOPLINES
- Medicare is set to negotiate prices for certain high-cost drugs with pharmaceutical manufacturers.
- To reduce prescription drug costs for Medicare beneficiaries, the Centers for Medicare and Medicaid Services has mapped out the negotiation process for the agency and drug manufacturers.
The Inflation Reduction Act, signed by President Biden in August 2022, gives the Centers for Medicare and Medicaid Services (CMS) the authority to negotiate prescription drug prices for certain high-cost drugs in Medicare. When this provision takes effect in 2026, it will mark the first time the federal government negotiates with drug manufacturers on behalf of millions of beneficiaries.
In July 2023, CMS established a negotiation process between the agency and drug manufacturers for the prices of drugs selected for negotiation. This voluntary process is designed to encourage manufacturers to come to the table and participate in a negotiation. However, drug manufacturers and other stakeholders with financial interest in the pharmaceutical industry are arguing that the law and CMS’s process facilitates government price setting instead of establishing a true negotiation process.
We believe CMS is establishing a process that facilitates trust and good-faith negotiations between the agency and drug manufacturers and challenges the notion that Medicare negotiation is akin to price setting.
CMS’s Process Aims to Engage Manufacturers in a Fair Negotiation
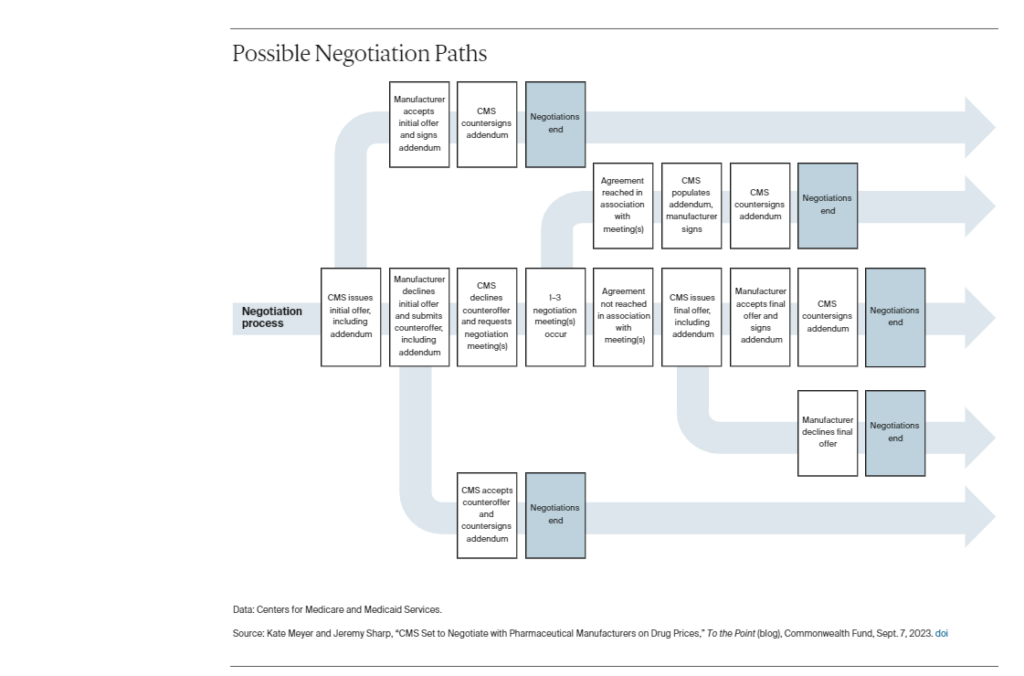
As the largest insurer in the U.S. health care system, CMS will have considerable leverage in negotiations. As such, manufacturers may employ various tactics to avoid negotiation, including attempting to game the system by submitting misleading or false information to CMS or filing lawsuits to stop negotiation from going into effect altogether. In an attempt to encourage manufacturer engagement in negotiations, CMS’s final guidance lays out a process for each manufacturer to engage in a fair and successful negotiation.
Prior to Negotiation: October 2023–January 2024
Prior to negotiation, CMS will host meetings with manufacturers to discuss information submitted to the agency regarding each drug.1 These meetings will provide manufacturers with an opportunity to provide context to CMS and better understand the agency’s positioning before heading into the negotiation process. CMS also will share nonproprietary information submitted by interested parties with the manufacturers, when feasible. These meetings will facilitate informed communications between CMS and manufacturers and set the stage for negotiations starting in February 2024.
Back-and-Forth Negotiation: February–August 2024
The process developed by CMS establishes back-and-forth negotiations that allows for engagement between the agency and manufacturers. CMS developed a negotiation process that involves multiple engagements between the agency and drug manufactures to find agreement on a negotiated price between February and August 2024.
By February 1, CMS will send manufacturers initial offer prices in written form. The manufacturer can either accept the initial offer or produce a written counteroffer within 30 days.
From there, CMS can either accept the manufacturer’s counteroffer or request negotiations. CMS will host three in-person or virtual negotiation meetings with manufacturers to reach agreement on final prices. At any point, CMS and manufacturers can settle on a price by signing an addendum to their initial agreement, which will end the process.
If negotiations end without an agreement, CMS will make a final written offer, which a manufacturer can either accept or reject. If a manufacturer is not satisfied with CMS’s final offer price the manufacturer can choose whether to sell its products in Medicare at the CMS price, sell at its preferred price and be subject to an excise tax, or withdraw its products from Medicare and Medicaid formularies, per the statute’s provisions. In fact, they have this choice at any point in the process.
During the negotiation process, CMS will keep all conversations with drug manufacturers confidential, which is a notable departure from the agency’s normal price-setting process for medical services. Maintaining confidentiality during the process will contribute to the integrity of negotiations and allow for more honest and direct conversation about fair prices. Confidentiality is common practice in other negotiations between government and industries — for example, negotiations between the Food and Drug Administration and drug manufacturers over user fees.
After Negotiation: September 2024–January 2026
Although CMS will keep information confidential during the process, the agency plans to publish an explanation of the negotiations — including how the two parties agreed upon a final price for each drug — after negotiation is complete and by March 1, 2025. Manufacturers can provide their own public account after the final agreement is signed. This will produce a public record of the dialogue between CMS and manufacturers and document negotiations. This transparency should also build public and policymaker confidence in the negotiation process.
Conclusion
CMS guidance facilitates a clearly structured, multistep Medicare drug price negotiation framework for drug manufacturers willing to participate in the program in good faith. This is a fundamentally different approach from other CMS processes for determining reimbursement. The parameters allow CMS to engage in dialogue with participating manufacturers that will result in lower prescription drug costs for millions of Medicare beneficiaries.
About the Authors
Kate Meyer – Director of Health Policy, Waxman Strategies
Jeremy Sharp – Vice President, Waxman Strategies
Originally published at https://www.commonwealthfund.org.