Leaders at the European Union’s Innovative Medicines Initiative are developing a large-scale multi-stakeholder international ecosystem to incorporate patient-reported outcomes and measures to improve patient engagement and drive value.
NEJM
By Tanja Stamm, PhD, Dr. rer. biol. hum., Mag. phil., MSc, MBA; Nick Bott, PsyD; Rob Thwaites, MA; Erika Mosor, MSc, PhD; Margaret R. Andrews, MPH; Joris Borgdorff, PhD; Yolima Cossio-Gil, MD, MPH; Simona de Portu, PharmD, MsC; Marc Ferrante, MD, PhD; Felix Fischer, PhD, et al.
June 9, 2021
Summary
Sustainable health care systems should be focused on outcomes instead of reimbursing for the services provided.
- Critical levers for accelerating this are patient engagement and a more comprehensive, standardized collection of patient outcomes.
- Also, more expertise is required on how these data can best be incorporated into the care process.
The Innovative Medicines Initiative project, a public-private partnership in the European Union, has launched Health Outcomes Observatory (H2O), a multi-jurisdictional ecosystem to incorporate patient-reported and other health outcomes into health care decision-making across Europe.
This initiative will initially focus on diabetes, cancer, and inflammatory bowel disease, evaluating and selecting meaningful outcome standards in a way that ensures broad acceptability among all stakeholders.
H2O will
- provide digital tools for patients and
- implement a state-of-the-art governance system that gives patients autonomy to control their data flows and
- allows for ethical data sharing,
- while enabling value-based care and driving better outcomes for patients.
Introduction
Although patients have a strong interest in the measurement of health outcomes of significance — such as symptom severity or functional status over time — health systems across Europe do not broadly engage in the measurement of such long-term health outcomes. Consequently, patient engagement in collecting and using relevant health outcomes data and information remains an underutilized strategy for incentivizing value-based care (VBC) in clinical practice.-
Patient outcomes and their health care experience can be improved through the systematic capture and appraisal of their perspectives. Patient-reported outcomes (PROs) reflect this patient perspective and are critical for delivering patient benefit and empowerment.,Today, health systems do not utilize all of the information they could gain from PROs to accurately measure and improve health care quality from the patient perspective.-
In a patient-centric VBC model, patients should be enabled to collect their outcomes data and have access and autonomy to use their health data in meaningful ways, such as self-managing their health in partnership with their health care providers. At the same time, data that includes patient perspectives should be made available and considered for health policy decisions. Although attracting more multi-stakeholder interest, value-based models remain insufficiently researched and not implemented on a wide scale.
Measurement of Value Is Critical for Sustainable Health Care Systems
Health care costs absorb a significant proportion of national gross domestic product globally, ranging between 3.1% (Indonesia) and 16.9% (United States) in Organisation for Economic Cooperation and Development (OECD) countries. Moreover, it is suggested that as much as 20% of health care spending is being wasted in these countries. There is increasing evidence and acceptance that health care financing should be focused on outcomes rather than on reimbursing for the services provided to achieve a sensible allocation of sparse resources. This shift from volume to value requires the design, development, and deployment of products, services, and integrated solutions that deliver value by improving patient outcomes in efficient and effective ways. Health systems need to become more sustainable in the face of challenges (such as an aging society) and to take full advantage of new personalized and targeted therapies. While reimbursement and funding decisions are based on health technology assessment approaches, a broader approach to VBC — seeking to demonstrate continuous improvement through regular assessments of effectiveness, impact, and outcomes at a system level — remains underdeveloped.,
In a patient-centric VBC model, patients should be enabled to collect their outcomes data and have access and autonomy to use their health data in meaningful ways, such as self-managing their health in partnership with their health care providers.At the same time, data that includes patient perspectives should be made available and considered for health policy decisions.
To enable this, one would need large-scale and broad-ranging outcomes data that are currently lacking. As a result, value-based models today are often implemented as pilots, lack scalability, are somewhat simplistic, and do not convince the skeptics.
Patients’ Voices Are Insufficiently Considered
Patients’ views on health outcomes are rarely considered at the individual or clinical condition level. As defined by patients, outcomes include their level of satisfaction with their current health status and quality of life, being capable of performing daily activities, having adequate relief from symptoms such as pain and fatigue, and seamlessly managing their health care within their daily lives. Consideration of the patient perspective is not only ethically desirable, but it is also fundamental to improvement in overall patient outcomes. In cancer care, there is increasing evidence that when self-reported symptom monitoring was integrated with clinical management, the clinical benefits, including increased survival, were observed.,
To make the subjective patient perspective an objective measure, both generic and disease-specific standards have begun to emerge. For example, the International Consortium for Health Outcomes Measurement ( ICHOM) publishes outcomes standards for multiple health conditions and collaborates with the OECD on collection, analysis, and publication of PROs for international comparison. The U.S. National Institutes of Health Patient-Reported Outcomes Measurement and Information System ( PROMIS) develops validated instruments that offer computer adaptive testing and include scores that allow for comparison to normative samples. However, the uptake of these standards at scale remains limited. There are very few examples of patient-focused value assessments (PROs and patient experience measures) being routinely implemented in individual clinical care settings at scale.
There are several reasons for this stagnation. There is a lack of best-practice models and infrastructure for efficiently capturing, managing, and utilizing PROs in clinical practice. Furthermore, standardization and interoperability of outcome measurement schemes are still insufficient, which means they are not comparable between providers, health systems, or countries. For example, more than 280 measures have been developed to assess depression. Only recently have psychometric methods been used to distinguish between PROs as underlying constructs (such as physical function, pain, or depression) and the respective instruments to assess those constructs.-If outcomes are collected variably and on a small scale, only small pockets of evidence are generated with limited generalizability, and their impact on clinical decision-making remains minimal.
The H2O Project: A Novel Approach to Incorporate Patient-Reported Outcomes Measurement
H2O is a new public-private consortium that brings together patients, patient advocates, clinicians, health care providers, academic researchers, and scientists and executives from the life sciences, pharmaceutical, and medical device industries. It will establish a multinational ecosystem to collect and incorporate patient-reported outcomes and other health outcomes into health care decision-making. The consortium will appraise and select outcome sets, ideally from among existing and published standards, in a way that ensures broad acceptability by patients, health care providers, and regulators to achieve meaningful outcome measurements at scale in three pilot disease areas: diabetes (types 1 and 2), inflammatory bowel disease, and oncology (with an early focus on lung and breast cancer), with a plan to expand into other areas.
There is a lack of best-practice models and infrastructure for efficiently capturing, managing, and utilizing PROs in clinical practice. Furthermore, standardization and interoperability of outcome measurement schemes are still insufficient, which means they are not comparable between providers, health systems, or countries.
To achieve this, we set up a rigorous process using a Delphi exercise,,which focuses primarily on the feasibility of implementation of the set, the user-friendliness of the methodology used, and the possibility for patients to report their outcomes as independently as possible, and by leveraging technologies. In the first Delphi round, representatives from relevant stakeholder groups, including patients, subject-matter experts, providers, regulators/authorities, and scientists and executives from the life sciences industry ( Table 1), commented and provided recommendations to the initial draft of six methodological steps.
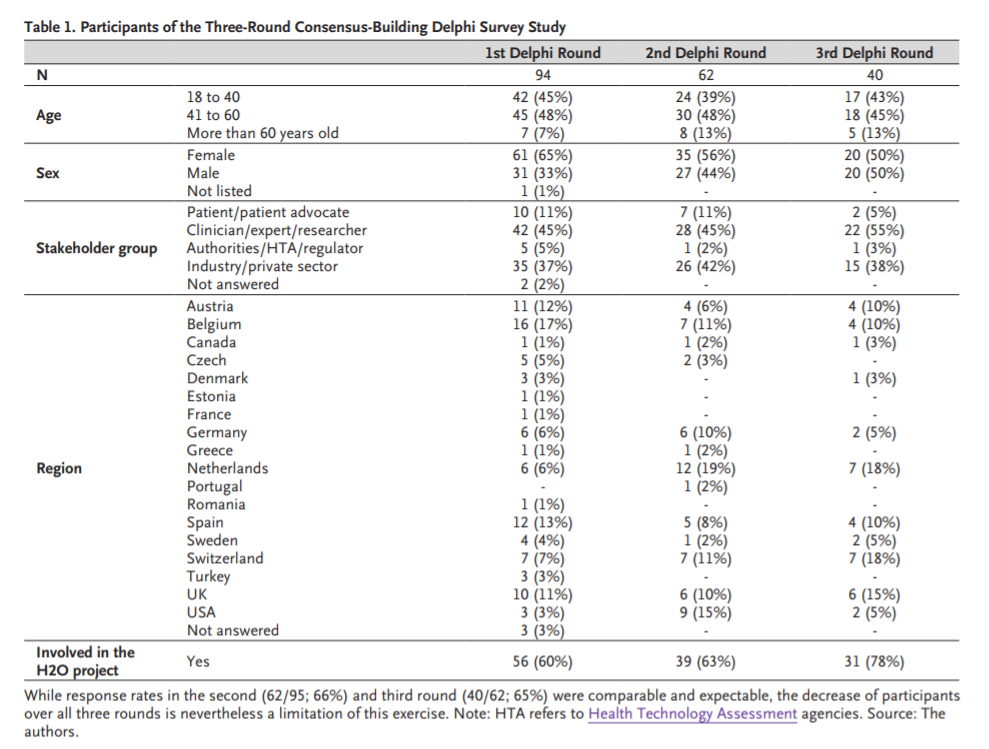
In the second round of the Delphi exercise, participants could add comments on the refined draft and scored their level of agreement with each step on a scale of 1 to 10, with 1 indicating no agreement and 10 indicating complete agreement. In the third round, we shared with the participants the results from the scorings in the second round separately for all four stakeholder groups and asked them to vote again on the process steps. A threshold value of 7 on the agreement scale was utilized for retaining a step. The final agreed process steps included (i) establishing a core team, (ii) performing a stakeholder mapping and identifying a broader reference group, (iii) starting a more comprehensive endorsement process, e.g., by engaging the relevant scientific societies, (iv) reviewing and critically appraising the existing standards using a literature review, (v) conducting a Delphi study with the broader reference group identified earlier, and (vi) holding a final consensus conference (Appendix).
Levels of agreement for each step were all above the threshold value of 7, ranging from 7.5 to 10. We will follow this process in each disease area. The agreed-upon outcomes and measures then constitute the basis for the data collection in the Observatories. The selected outcome sets will consist of existing standards, such as the ICHOM sets, generic elements, and digital tool data. Regarding PROs, we plan to longitudinally ask patients a set of basic questions on general well-being and provide options to report more symptoms and side effects, if applicable. The focus of our effort will be to identify practical and patient-friendly solutions to enable patients to record and report outcomes and side effects with minimum effort. When H2O is scaled up to address other disease areas in the future where no core outcome sets exist, we plan to commission existing organizations to work with us in the development of new core outcome sets.
H2O is a new public-private consortium that brings together patients, patient advocates, clinicians, health care providers, academic researchers, and scientists and executives from the life sciences, pharmaceutical, and medical device industries. It will establish a multinational ecosystem to collect and incorporate patient-reported outcomes and other health outcomes into health care decision-making.
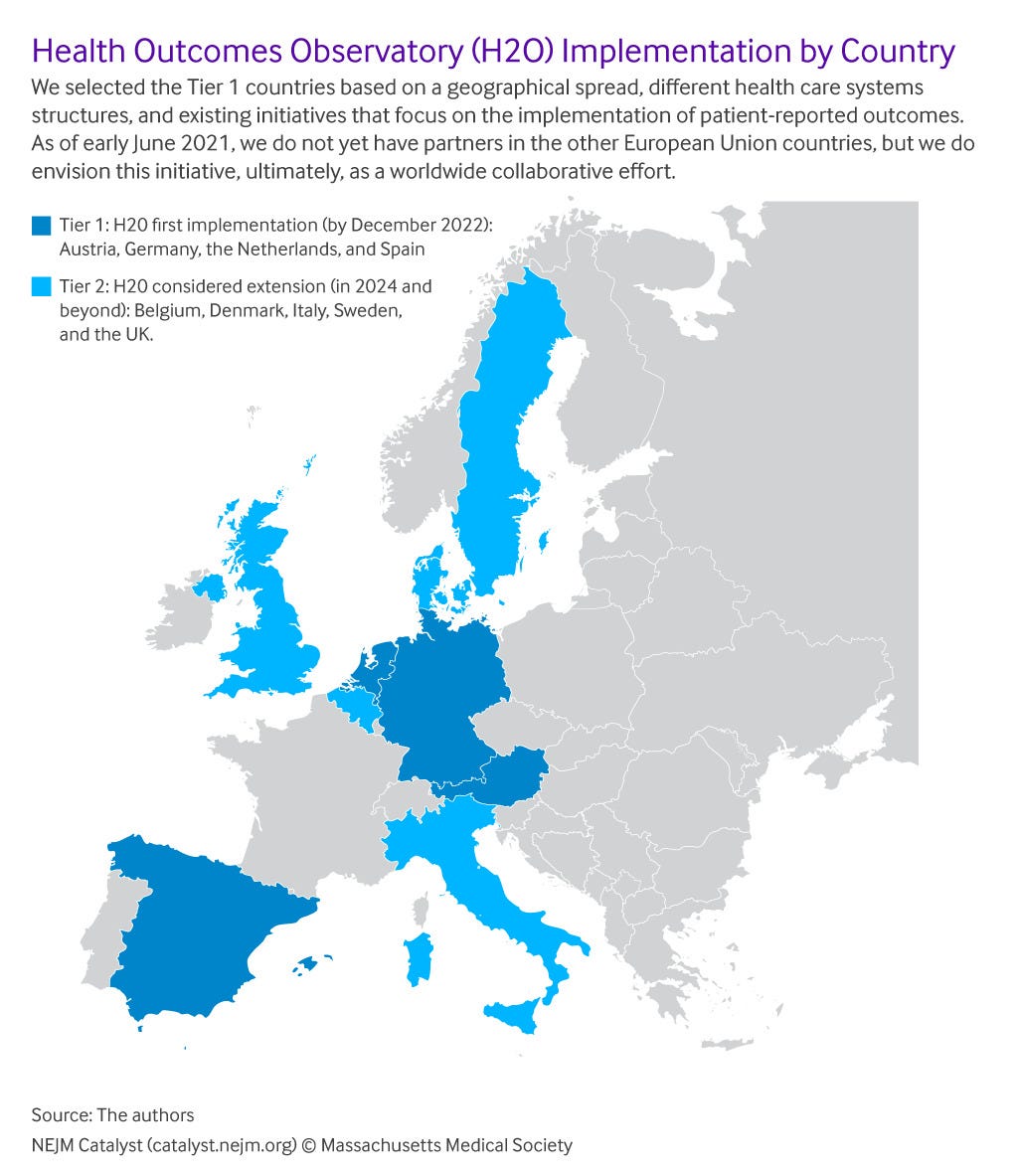
H2O will set up independent entities (national Observatories) initially in Austria, Germany, the Netherlands, and Spain (), to be operational by the end of 2022 and integrated into the national health care ecosystems. These four were selected in part because of the diversity of geography and of the health care systems.
Figure 1
We will deploy a federated architecture for data collection, management, and analysis combined with a centralized PROs data collection infrastructure in each country. We will start from the formalized outcomes definition, including its semantics (e.g., value lists), and then map this to the Observational Medical Outcomes Partnership ( OMOP) Common Data Model to ensure data interoperability. Analytic code will travel to where the data resides, and results will be sent back (Figure 2).
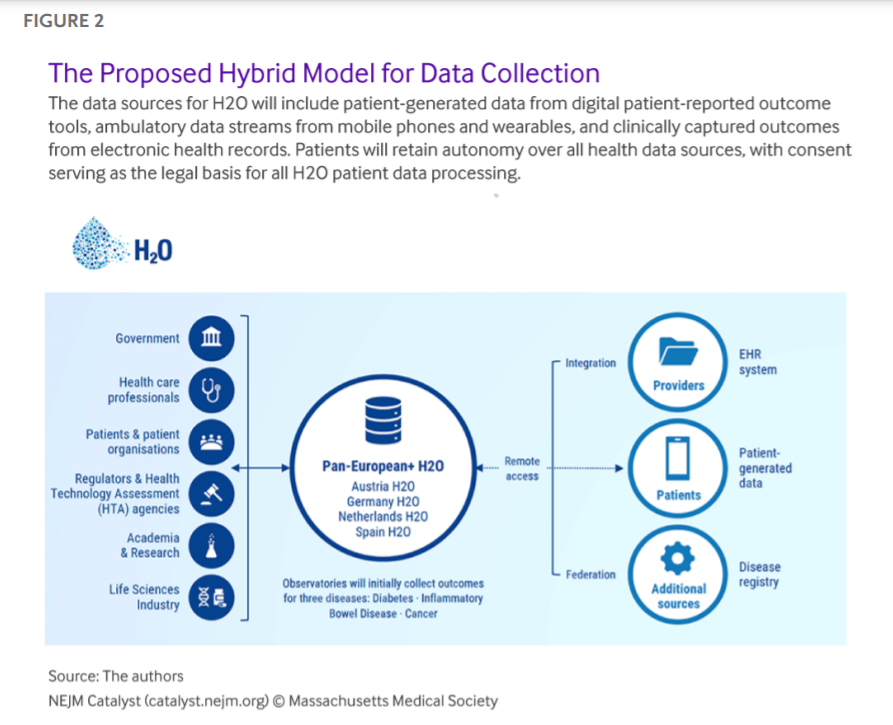
While clinical data will remain at each health care site — safeguarded under its local and respective national legal frameworks — the Observatories will manage the data (whether actively reported by or passively collected from patients who agree to use wearables or other monitoring tools) that come directly from the patients on their behalf and through their consent. H2O aims to establish a culture of continuous outcome measurement across the health care ecosystem, placing a strong emphasis on the need for and importance of linking PROs to the corresponding clinical outcomes measurement sets and incorporating these into the process of care. A pan-European Observatory will also be founded to connect and oversee the national Observatories’ activities to ensure alignment of the operating principles and core outcome sets to allow aggregating data at the European level.
Patients as Health Outcomes Data Controllers
Patients will be able to join H2O independently of their health care providers. Digital technologies will allow patients to submit their data via standardized questionnaires on their smartphones or other devices. Patients will use a personalized dashboard with information on their disease progression, treatment, and outcomes. While patients in some countries already have access to their electronic health data, their awareness and willingness to share this information remain the exception, not the rule. The H2O technology will facilitate this with patient- and provider-friendly visualizations to improve communication and exercise more self-control and self-management.
To address the concerns of health care providers and patients and to build trust on both sides, we will implement data protection by design and default principles. We will also work closely with health care providers to ensure that data from the Observatories will provide additional clinical value. The data extraction process run by a health care provider or hospital will facilitate each national Observatory’s population. Patients will have to consent to permit the communication of personal data between the providers and the Observatories. Furthermore, they will choose the health care providers with whom they wish to share relevant outcomes data.
H2O aims to establish a culture of continuous outcome measurement across the health care ecosystem, placing a strong emphasis on the need for and importance of linking PROs to the corresponding clinical outcomes measurement sets and incorporating these into the process of care.
We recognize that patient engagement is critical. We will, therefore, involve patients from the beginning, on all levels, including the steering groups. The aim is to co-create with patients to make something useful for them, to empower them with data, and to make their communication with their clinician more valuable.
Health Data Will Be Safeguarded as an Essential Resource
This Health Outcomes Observatory initiative goes by the name H2O. Like water, anonymized and aggregated health data should be a natural resource available to anybody who has a legitimate need to use it while complying with existing regulations, such as the General Data Protection Regulation (GDPR) which the European Union put in place on May 25, 2018. Health data includes PROs, which may be especially important for individual patients, e.g., to improve communication with clinicians, but also for patient organizations ( Table 2). However, much like water, certain types of health data need to be appropriately safeguarded and managed in society’s interests.
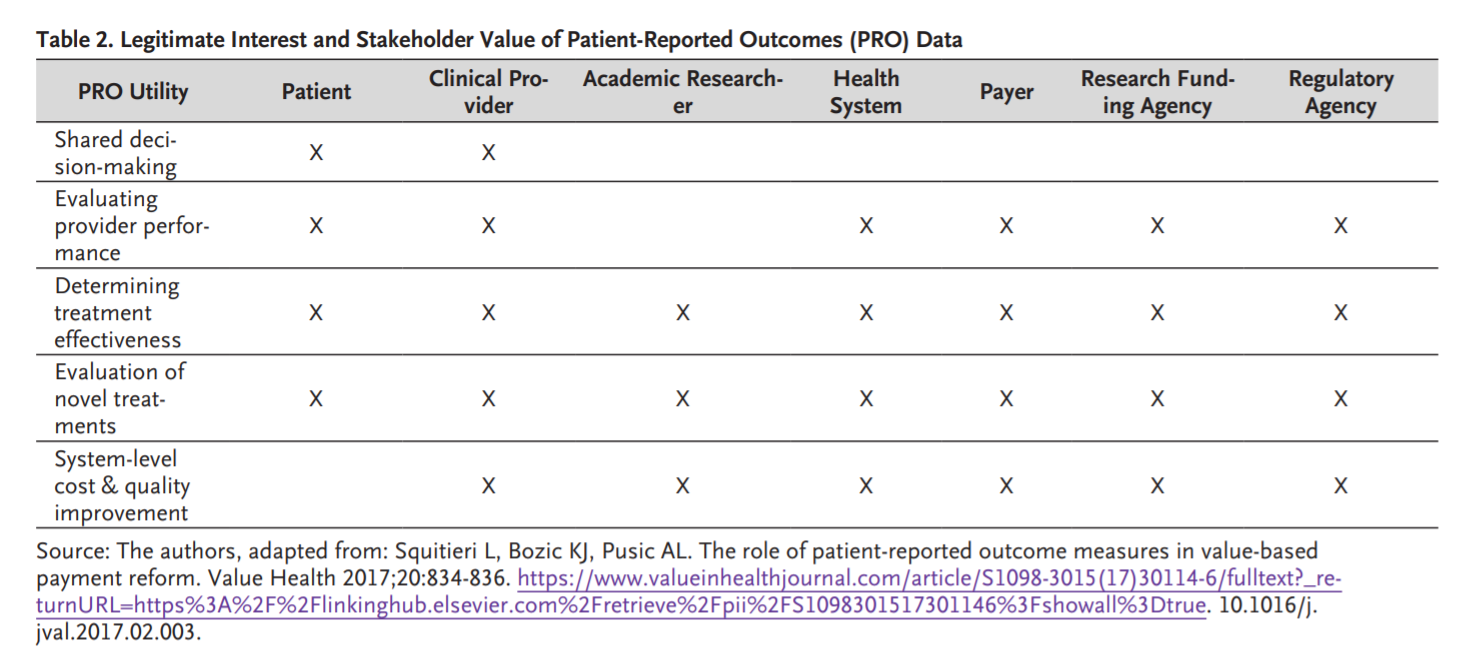
Personal health data are a non-replicable asset that one cannot recreate or find elsewhere. Access to these data can be a crucial prerequisite for innovation in research and the development of new treatments, devices, products, and therapies. Recently, the topic has been introduced in a proposal of the European Commission for the Regulation on European Data Governance, where suggestions have been put forward to foster the availability of data for use by increasing trust in data intermediaries and by strengthening data-sharing mechanisms across the EU.
H2O will be built using a non-for-profit model to allow health data to become broadly available in an ethically and legally appropriate manner. The non-for-profit governance model we are developing and implementing will be similar to the governance models used by utilities in several countries to manage essential resources, e.g., for purifying and distributing water. H2O’s nature as a public-private partnership representing all stakeholders’ perspectives, is built upon a robust data protection foundation that gives complete control of the data to the patients and will enable open, fair, and objective data sharing.,
Looking Ahead
H2O is a catalytic project that will provide real-world, high-quality outcomes data to patients, health care providers, and other stakeholders who have a legitimate interest in using them for permitted and socially acceptable purposes. Co-creation will be used throughout, ensuring a multi-stakeholder approach on all levels of conceptualization and deployment. H2O will thus enable VBC and ultimately drive better outcomes for patients.
About the authors
Tanja Stamm, PhD, Dr. rer. biol. hum., Mag. phil., MSc, MBA;
Professor and Head of Section for Outcomes Research, Vice Head of the Center for Medical Statistics, Informatics and Intelligent Systems,
Medical University of Vienna, Austria; Group leader, Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Vienna, Austria
Nick Bott, PsyD;
Global Head, Bioethics, Technology Ethics & Responsible Innovation,
Takeda Pharmaceuticals, Cambridge, Massachusetts, USA
Rob Thwaites, MA;
Founder and Director, Albion House Consulting Ltd, Marlow, United Kingdom;
Erika Mosor, MSc, PhD;
Senior Researcher, Section for Outcomes Research,
Medical University of Vienna, Austria
Margaret R. Andrews, MPH; Public Health Researcher, Section for Outcomes Research, Medical University of Vienna, Austria
Joris Borgdorff, PhD; Software Engineer, The Hyve B.V., Utrecht, the Netherlands;
Yolima Cossio-Gil, MD, MPH; Associate Director Evaluation & Data Management, Vall d’Hebron University Hospital, Barcelona, Spain
Simona de Portu, PharmD, MsC; Director Health Economics, Reimbursement and GA Diabetes, EMEA region at Medtronic,
Medtronic International Trading Sarl, Tolochenaz, Vaud, Switzerland
Marc Ferrante, MD, PhD;
Assistant Professor, KU Leuven University Hospitals Leuven, Leuven, Flanders, Belgium
Felix Fischer, PhD;
Research Fellow, Charité — Universitätsmedizin Berlin, Germany
Farhan Hameed, MD, MS, FHIMSS, FAMIA;
Vice President, Global Real World & Commercial Data — Strategy, Analytics & Insights,
Pfizer, New York, New York, USA
Jan Hazelzet, MD, PhD;
Professor Em. in Healthcare Quality & Outcome, Erasmus MC, Rotterdam, Zuid-Holland, the Netherlands
David Hopkins, BSc(Hons), MBChB, MRCP, FRCP;
Consultant Diabetes Physician and Clinical Director for Outpatient Medical Specialties, Department of Diabetes, School of Life Course Science, King’s College London, United Kingdom
Dipak Kalra, MBBS, PhD;
Professor of Health Informatics at University College London, United Kingdom; Visiting Professor of Health Informatics, University of Gent, Belgium; President, The European Institute for Innovation through Health Data, Brussels, Belgium
Thomas Metcalfe, BSc, MBA;
Data Policy Leader, Personalised Healthcare, F Hoffmann-La Roche AG, Basel, Switzerland
Eva Molero, LLM;
Founder and Chief Executive Officer, Teamit Research, Barcelona, Spain
Rachel Newson, PhD;
Principal Research Scientist, Global Patient Outcomes and Real World Evidence,
Eli Lilly and Company, Utrecht, the Netherlands
Francesco Patalano, MD;
Head of Pediatric and Patient Reported Outcomes Center of Excellence, Novartis International AG, Basel, Switzerland
Fabian Prasser, PhD;
Head of Medical Informatics Group, Charité — Universitätsmedizin Berlin, Germany; Corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Germany; Full Professor of Medical Informatics, Berlin Institute of Health, Berlin, Germany
Matthias Rose, MD, PhD;
Professor and Chair, Center for Internal Medicine and Dermatology, Department of Psychosomatic Medicine, Charité — Universitätsmedizin Berlin, Germany
Mikkel Lindskov Sachs, MS;
Head of Strategic Communication, Trial Nation Denmark, Copenhagen, Denmark
Jeannette Soderberg, PhD;
Director European Research, JDRF International, Stockholm, Sweden
Valentina Strammiello, MA
Head of Programmes, European Patients’ Forum, Brussels, Belgium
Lonneke van de Poll, PhD
Group Leader, Division of Psychosocial Research and Epidemiology, and Survivorship theme leader,
The Netherlands Cancer Institute, Amsterdam, the Netherlands; Senior Researcher, Department of Research, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands
Meni Styliadou, LLM
VP Distinguished Fellow, Data Science Institute, Takeda R&D, Zurich, Switzerland
References
See the original publication.
Originally published at https://catalyst.nejm.org on June 9, 2021.
To download the PDF, open the URL below:
https://catalyst.nejm.org/doi/pdf/10.1056/CAT.21.0146