health strategy institute (hsi)
review
management, engineering and
technology review
Joaquim Cardoso MSc.
Senior Research and Strategy Officer (CRSO) —
for the Health Strategy Research
Chief Editor — for the Health Strategy Review
July 27, 2023
Abstract
Lung cancer remains one of the most life-threatening diseases and is currently managed through invasive approaches such as surgery, chemo- or radiotherapy.
In this work, the authors introduce a novel method for the targeted delivery of a therapeutic laser for the treatment of tumors in peripheral areas of the lungs.
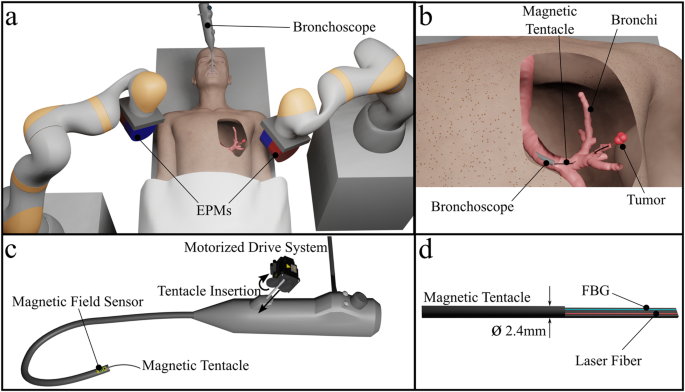
- The approach uses a 2.4 mm diameter, ultra-soft, patient-specific magnetic catheter delivered from the end of a standard bronchoscope to reach the periphery of the lungs.
- Integrated shape sensing facilitates supervised autonomous full-shape control for precise navigation into the sub-segmental bronchi, and an embedded laser fiber allows for treatment via localized energy delivery.
The authors report the complete navigation of eight primary lumina in the bronchi of an anatomically accurate phantom (developed from computed tomography (CT) data) and successful laser delivery for photothermal ablation.
The authors further evaluate the approach in three diverse branches of excised cadaveric lungs, showing a mean improvement in navigation depth of 37% with less tissue displacement when compared to a standard semi-rigid catheter and navigation depth repeatability across all tests of <1 mm.
DEEP DIVE
Personalized magnetic tentacles for targeted photothermal cancer therapy in peripheral lungs — Communications Engineering
Communications Engineering
Giovanni Pittiglio, James H. Chandler, Tomas da Veiga, Zaneta Koszowska, Michael Brockdorff, Peter Lloyd, Katie L. Barry, Russell A. Harris, James McLaughlan, Cecilia Pompili & Pietro Valdastri
July 2023
Introduction
Lung cancer has the highest worldwide cancer mortality rate, with 130,000 deaths projected for 2022 in the US alone1( https://seer.cancer.gov/statfacts/html/lungb.html). In early-stage non-small cell lung cancer (NSCLC), which accounts for around 84% of cases, curative surgical intervention is the standard of care. However, surgery is typically highly invasive, necessitating the removal of a large portion of lung tissue which is not suitable for all patients and may impact resulting lung function. Furthermore, although diagnosis as part of lung cancer screening programs has demonstrated survival benefit, it has also highlighted the urgent need to find non-invasive and scalable methods to achieve early diagnosis and therapy2.
Initial screening via medical imaging techniques such as X-ray and computed tomography (CT) will typically require tissue biopsy to confirm the diagnosis. Biopsy sampling may be performed percutaneously using rigid needles or from inside the airways using a semi-rigid endoscope (bronchoscope) and specialized sampling tools3. Sampling via a bronchoscope is beneficial as it removes the need to puncture the pleural membranes; however, it is limited in reach due to the size of the bronchoscope (typically 5–6 mm)4,5; allowing access down to the second generation of the bronchi via camera-based surgeon-driven navigation (bronchoscopy). To explore the deeper anatomy, electromagnetic navigation (EMN) can be used, where a pre-bent passive catheter is inserted down the bronchoscope’s tool channel and is steered by rotating around its axis from the proximal end. EMN is performed based on a pre-operative CT scan of the lungs and provides surgeons with active localization within the bronchial tree. However, the pre-bent shape, rigidity, and size of EMN tools (around 2.7 mm diameter, Edge™ EWC Firm Tip Medial Procedure Kit, Medtronic) may have a disruptive effect on the targeting process as poor conformation of the catheter to the anatomy and associated tissue deformation impact the ability to target the tumor based on a pre-operative image of non-deformed anatomy.
EMN extends standard bronchoscopy, normally reaching the second generation of the bronchi, to the fifth generation. This equates to a 30% increase in the navigation of the bronchial anatomy.
With the aim of improving tool dexterity and anatomical reach, robotic approaches to endoscope and catheter navigation have been proposed6. Current commercial systems include the MONARCH (Auris Health, Inc), the Ion (Intuitive Surgical) and the Galaxy system (Noah Medical). These systems are designed to steer only the tip of the catheter, so there is no guarantee that proximal anatomy is not deformed during navigation. Additionally, relatively large diameters are retained (MONARCH 4.2 mm diameter; Ion and Galaxy 3.5 mm diameter), which limits potential anatomical reach. Alongside commercial robotic bronchoscopy systems, several research platforms have been proposed. These include a tendon-driven continuum robot7, concentric-tube actuated steerable needles with design optimization and path planning for lung lesion targeting8, a one-degree-of-freedom soft pneumatically driven robot for deployment from a standard bronchoscope9,10, and anatomy-specific fully magnetic catheters for shape-forming navigation11.
In contrast to other approaches, magnetic actuation is remote, reducing or removing the reliance on proximal mechanical and pneumatic connections and facilitating miniaturization. Indeed, tip-driven magnetic catheters have been demonstrated for cardiac and neurovascular applications at scales down to 0.4 mm12. However, designs that rely on axial magnetization and thus control of the tip limit the possibility for shape forming and must instead depend on the deformation of the soft structure during interaction. With a generation of appropriate magnetic fields and gradients, it is possible to control up to eight DOFs13, making it viable to largely improve shape-forming and deliver non-disruptive navigational capabilities of magnetic catheters beyond existing tip-driven designs. We demonstrated successful navigation of anatomy-specific fully magnetic catheters-namely magnetic tentacles11. This approach presents the advantages of being: (1) specific to the anatomy and guaranteed to succeed via pre-operative planning; (2) miniaturized-2.4 mm diameter; and (3) softer than the anatomy and fully-shape controllable via magnetics. These three main features have the potential to revolutionize navigation inside the anatomy. In fact, in contrast to taking advantage of functional contact, as the majority of tip-actuated catheters would, our method facilitates shaping along the length. This enables follow-the-leader motion, thus, eliminating the need for tissue interaction during introduction.
With improved non-disruptive navigation, it is also possible to move beyond biopsy and assist in unlocking the potential of targeted minimally invasive therapies, acting only on malicious cells while allowing healthy tissue and organs to continue normal function. Laser ablation is a minimally invasive technique that is used to treat a range of early cancers, such as skin, penile and esophageal14, and can be given as either primary treatment or as adjuvant therapy. In the treatment of early-stage NSCLC, ablation techniques such as laser ablation have not outperformed stereotactic body radiation therapy, despite offering a non-ionizing technique for treatment15. The limited effectiveness of standard laser ablation techniques could be due to the light delivery method employed, which is through the insertion of fiber optics using needles and is indiscriminate in the heating of healthy and tumorous tissue. To help address this localization issue, the use of plasmonic gold nanoparticles that strongly absorb laser energy at a specific wavelength of light has been investigated to increase heating in tumor tissue while sparing healthy tissue16. Furthermore, as these particles can be molecularly targeted with tumor-specific markers, such as epidermal growth factor receptor (EGFR), they can provide further tumor localization when introduced systemically into the body. Due to the rapid attenuation of laser light in tissue, illumination sources need to be in close proximity to the tumor, which is commonly achieved through needle insertion under image guidance17. When performed in a minimally invasive fashion, i.e., endoscopically, this can reduce pain, discomfort, and recovery time for the patient. In this case, robotics has the potential to improve precision and safety18,19.
In this paper, we advance the capability and application of magnetic tentacles, which we previously introduced11, for use in targeted photothermal therapy (PTT). We present an optimization technique that can deliver a unique tentacle design capable of non-disruptive navigation to multiple targets (e.g., tumors) deep within the same anatomical structure, making multi-target therapy possible. To guarantee the target tumor is reached, we introduce supervised autonomy into our robotic platform based on real-time localization. The magnetic tentacle is synchronously inserted and magnetically manipulated to conform to the anatomy in an open-loop fashion based on pre-operative planning and path optimization. Tentacle position and shape are fed back in real-time to the clinician for monitoring the procedure.
We present a tracking method based on a magnetic localization of the bronchoscope and full-shape reconstruction of the tentacle via a Fiber Bragg Grating (FBG) sensor. Information from both sensors is combined and overlayed with the segmented pre-operative CT scan for visualization purposes. A fabrication technique is proposed to deliver integrated sensing and laser fibers into a 2.4 mm diameter tentacle with an optimized lengthwise magnetization profile.
In the present paper, we demonstrate successfully supervised autonomous navigation of magnetic tentacles in the most complex lumina within a bronchial phantom along diverse anatomical structures in both the left and right bronchial tree. We demonstrate the possibility of deploying 1064 nm laser light at powers sufficient to cause heating and show improved targeting due to the combination of accurate navigation and therapy. Navigation demonstrations are extended to a cadaveric lung specimen, where we show an exploration of the left bronchial tree and compare the results with a standard EMN catheter. Results demonstrate the possibility for deeper navigation into the lungs while imparting less deformation on the surrounding anatomy. While the nominal diameter of the tentacle is comparable to standard tools, thus the same depth should be achieved; our experiments demonstrate an overall improvement of 37% in navigation depth.
The presented results represent a potential first step toward transforming the treatment of cancer in peripheral areas of the lungs via a more accurate, patient-specific, and minimally invasive approach.
Results
See the original publication (this is an excerpt version)
Discussion
In the present work, we introduce a novel approach to targeted therapy for minimally invasive lung cancer treatment. This technique has two main components: (1) a 2.4 mm diameter magnetically actuated patient-specific flexible catheter and (2) laser fiber-enabled targeted therapy. The former is based on the fabrication, localization, and full-shape control of magnetic tentacles with a specific lengthwise magnetization profile. These magnetic tentacles were optimized to the patient-specific bronchial tree and remotely actuated via collaborative control of two external permanent magnets. Laser delivery was successfully demonstrated on a tumor phantom containing gold nanoparticles. These particles can be functionalized to preferentially bind to specific tumor cells when introduced systemically and convey targeted treatment.
We present a platform capable of real-time tracking and actuation of the magnetic tentacles inside the anatomy and discuss the design and fabrication of the patient-specific tentacles. The tentacles were fabricated with an OD of 2.4 mm, which is comparable to the standard tools used in EMN. Therefore, they can enable reach to the fifth generation of the bronchi, i.e., 30% deeper than standard bronchoscopes. We demonstrated the navigational capabilities under supervised autonomy in two sets of experiments: one set in a transparent phantom and the second set in an excised specimen of human lungs. In the former, we show eight diverse navigations in both left and right bronchi-specifically, the ability to reach the sub-segmental bronchi (2–4 mm ID).
In the cadaveric lungs, we demonstrate successful navigation in three branches of the left bronchi, against the two achieved using a standard catheter, corresponding to a mean improvement in navigation depth of 37%. Moreover, we show minimal deformation of the lumen, which can maximize targeting capabilities based on pre-operative imaging.
We noticed that, while it has little influence on the navigation performance, moving the EPMs point-to-point may not always be safe. In fact, from the experiments in the phantom, we see that the robots may enter a zone reserved for the patient’s body. To limit this, we will investigate safe robot planning and use larger magnets, which can allow the EPMs to be positioned further away from the patient while guaranteeing a functional magnetic field.
As part of the tissue phantom experiments, we also demonstrate laser delivery and show selective heating of tumor phantoms with and without gold nanoparticles via a magnetic tentacle-delivered laser fiber, simulating the proposed treatment paradigm and its effect on the tumor and normal tissue regions. This proposed approach has the potential to optimize minimally invasive delivery of therapy, i.e., with a soft optimal navigation technique combined with therapy targeting the tumor only. Compared to existing alternatives, this may have a fundamental impact on the quality of life of treated patients, such as removing the need for stereotactic body radiation therapy24.
It is worth mentioning that testing the efficacy of selective heating in a cadaver model was not possible due to the impossibility of introducing plasmonic nanoparticles in a localized and controlled manner.
Open-loop control in a clinical setting could lead to some unwanted behavior during navigation and/or interactions with lung tissue due to errors in sensor readings and initial localization. Future work will focus on this while compensating for anatomical motion related to respiration and heart dynamics. We anticipate that, in this case, a high-performing closed-loop control scheme would be required. In this case, we will leverage pre-operative imaging and shape control based on FBG sensing25. To confirm that navigation in dynamic environments and selective tissue heating can be achieved, testing in a more realistic environment (i.e., in vivo animal model) will be pursued in future work.
Herein, we focused on a four-segment fully magnetized tentacle with maximum magnetic content along the length. However, we will investigate different distributions of the lengthwise magnetic content and optimize other mechanical parameters26. In addition, we will investigate an upgraded design protocol capable of deriving a globally optimal solution within the framework of these magnetic and geometric variations.
Methods
See the original publication (this is an excerpt version)
Authors and Affiliations
Department of Cardiovascular Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Giovanni Pittiglio
STORM Lab, Institute of Autonomous Systems and Sensing (IRASS), School of Electronic and Electrical Engineering, University of Leeds, Leeds, UK
Giovanni Pittiglio, James H. Chandler, Tomas da Veiga, Zaneta Koszowska, Michael Brockdorff, Peter Lloyd & Pietro Valdastri
Leeds General Infirmary-Leeds Teaching Hospital, University of Leeds, Leeds, UK
Katie L. Barry
Future Manufacturing Processes Research Group, School of Mechanical Engineering, University of Leeds, Leeds, UK
Russell A. Harris
Ultrasound Group, Institute of Autonomous Systems and Sensing (IRASS), School of Electronic and Electrical Engineering, University of Leeds, Leeds, UK
James McLaughlan
Leeds Institute of Medical Research (LIMR), University of Leeds, Leeds, UK
James McLaughlan & Cecilia Pompili
Cite this article
Pittiglio, G., Chandler, J.H., da Veiga, T. et al. Personalized magnetic tentacles for targeted photothermal cancer therapy in peripheral lungs. Commun Eng 2, 50 (2023). https://doi.org/10.1038/s44172-023-00098-9
Originally published at https://www.nature.com on July 27, 2023.