the health transformation
institute for continuous health transformation
and digital health
Joaquim Cardoso MSc
Chief Researcher, Editor and Senior Advisor
January 5, 2023
- Scientists at Brigham and Women’s Hospital have developed a new cell therapy that can both kill established tumors and prevent cancer from recurring by training the immune system.
- The therapy repurposes living tumor cells by engineering them with the gene editing tool CRISPR-Cas9 to release a tumor cell killing agent and make them easy for the immune system to identify.
- The therapy was tested in mice with advanced brain cancer and human immune cells, and was found to be safe, effective and applicable. Further testing and development is needed before the therapy can be used in patients.
ORIGINAL PUBLICATION (excerpt)

Bifunctional cancer cell–based vaccine concomitantly drives direct tumor killing and antitumor immunity
SCIENCE TRANSLATIONAL MEDICINE
KOK-SIONG CHEN , CLEMENS REINSHAGEN , THIJS A. VAN SCHAIK, FILIPPO ROSSIGNOLI ,PAULO BORGES, NATALIA CLAIRE MENDONCA, REZA ABDI ,BRENNAN SIMON ,DAVID A. REARDON, HIROAKI WAKIMOTO ,AND KHALID SHAH,
4 Jan 2023
Abstract
The administration of inactivated tumor cells is known to induce a potent antitumor immune response; however, the efficacy of such an approach is limited by its inability to kill tumor cells before inducing the immune responses.
Unlike inactivated tumor cells, living tumor cells have the ability to track and target tumors.
Here, we developed a bifunctional whole cancer cell–based therapeutic with direct tumor killing and immunostimulatory roles.
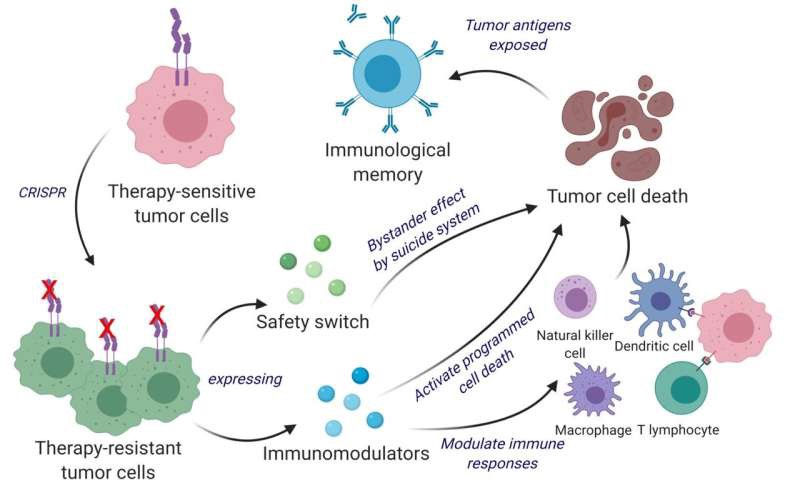
We repurposed the tumor cells from interferon-β (IFN-β) sensitive to resistant using CRISPR-Cas9 by knocking out the IFN-β–specific receptor and subsequently engineered them to release immunomodulatory agents IFN-β and granulocyte-macrophage colony-stimulating factor.
These engineered therapeutic tumor cells (ThTCs) eliminated established glioblastoma tumors in mice by inducing caspase-mediated cancer cell apoptosis, down-regulating cancer-associated fibroblast-expressed platelet-derived growth factor receptor β, and activating antitumor immune cell trafficking and antigen-specific T cell activation signaling.
This mechanism-based efficacy of ThTCs translated into a survival benefit and long-term immunity in primary, recurrent, and metastatic cancer models in immunocompetent and humanized mice.
The incorporation of a double kill-switch comprising herpes simplex virus–1 thymidine kinase and rapamycin-activated caspase 9 in ThTCs ensured the safety of our approach.
Arming naturally neoantigen-rich tumor cells with bifunctional therapeutics represents a promising cell-based immunotherapy for solid tumors and establishes a road map toward clinical translation.
These engineered therapeutic tumor cells (ThTCs) eliminated established glioblastoma tumors in mice by inducing caspase-mediated cancer cell apoptosis, down-regulating cancer-associated fibroblast-expressed platelet-derived growth factor receptor β, and activating antitumor immune cell trafficking and antigen-specific T cell activation signaling.
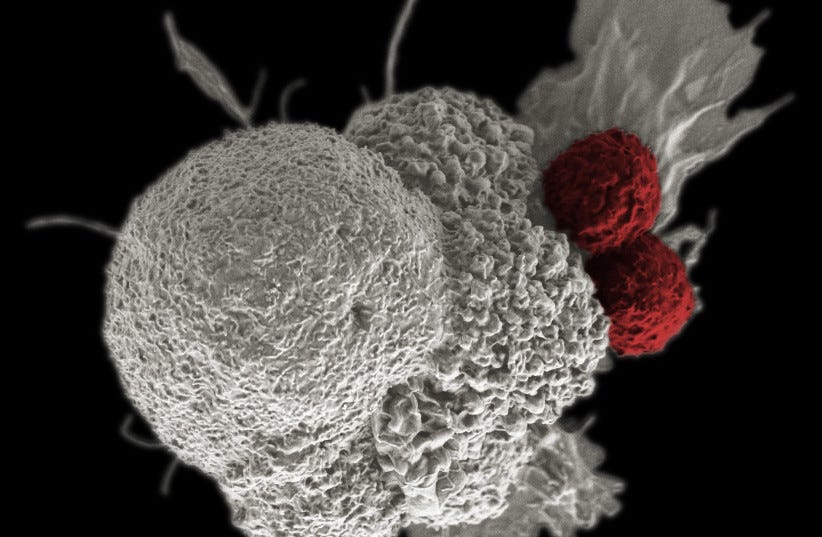
Turning tumors into turncoats
Inactivated tumor cells have been previously investigated for their anti-cancer ability and have only shown limited clinical benefit.
To overcome this, Chen et al. have engineered living tumor cells to secrete interferon beta (IFNB) and granulocyte-macrophage colony-stimulating factor (GM-CSF) to both target tumors and alter the tumor microenvironments.
These engineered therapeutic tumor cells (ThTCs) induced improved survival and long-term immunity in glioblastoma humanized mice.
In addition, Chen et al. incorporated a double kill-switch to prevent secondary tumor initiation to provide an effective and safe therapy that warrants further investigation. — DH
Inactivated tumor cells have been previously investigated for their anti-cancer ability and have only shown limited clinical benefit.
To overcome this, Chen et al. have engineered living tumor cells to secrete interferon beta (IFNB) and granulocyte-macrophage colony-stimulating factor (GM-CSF) to both target tumors and alter the tumor microenvironments.
Originally published at https://www.science.org