Results of the Second Brazilian Early Lung Cancer Screening Trial (BRELT2)
JCO Global Oncology
Bruno Hochhegger, MD, PhD1,2; Spencer Camargo, MD, PhD1; Gustavo Borges da Silva Teles, MD, PhD3; Rodrigo Caruso Chate , MD, PhD3; Gilberto Szarf , MD, PhD3; Marcos Duarte Guimarães , MD, PhD4; et al
January 24, 2022
Image: cedars-sinai
The Executive Summary below was edited by the author of the blog, as a voluntary christian service. The purpose here is to help the fight against cancer, and save lives.
Executive Summary (by Joaquim Cardoso MSc.)
- It is a well-known fact that in Brazil, the majority of lung cancer cases are diagnosed in advanced stages.2,17
- The present study demonstrated a significant stage shift, with 70% of cancers diagnosed as early stage.
The Second Brazilian Early Lung Cancer Screening Trial is the first multi-institutional LCS study in Brazil with 3,819 enrolled participants.
- The authors reported at JCO Global Oncology analogous outcomes of the main international studies confirming 2.1% prevalence of lung cancer.
- Lung cancer was diagnosed in 74 of 122 suspicious nodules (3.5% biopsy rate). Granulomatous diseases did not increase the number of biopsies.
- The reality of lung cancer in Brazil is similar to that found worldwide.
There are innumerous challenges for LCS in Brazil; however, it was possible to obtain satisfactory results in all regional scenarios.
- In Brazil, surgical treatment represents a much lower cost than other treatments with chemotherapy, radiotherapy, or agents such as immunotherapy or target therapy.
- Therefore, there is the possibility of cost reduction in the treatment of lung cancer in Brazil; however, we recognize that larger population-based screening programs and prospective studies are required to confirm this hypothesis.
Evidence of the effectiveness of LCS is clear worldwide.
In Brazil, difficulties in its implementation are related to
- the organization of the health system,
- access to CT scans and treatment methods,
- and cultural acceptance of the method slow down the process.
The greatest challenge of modern medicine, with the growing demand for high-cost technology, is finding ways to benefit many persons without exponentially increasing costs.
- This challenge embraces the need to integrate the private system’s activities with the public system, generating demand and access in an organized and structured way to various technologies already available in some regions.
- Another possible path to reduce costs in the health system is the verticalized organization, where all the health care, including prevention, diagnosis, and treatment, is organized as a single company. This kind of management favors screening and prevention programs because of the reduction of the high costs of treating advanced diseases.
The authors hope that it could stimulate the creation of organized screening programs in regions still endemic for tuberculosis and other granulomatous diseases.
ABSTRACT
PURPOSE
This paper aims to present the results of a series of several Brazilian institutions that have been carrying out lung cancer screening (LCS).
MATERIALS AND METHODS
This is a retrospective, cohort study, with follow-up of individuals of both sexes, with a heavy smoking history, who participated in LCS programs
- between December 2013 and January 2021 in
- six Brazilian institutions located
- in the states of São Paulo, Rio Grande do Sul, and Bahia.
RESULTS
Three thousand four hundred seventy individuals were included, of which 59.8% were male (n = 2,074) and 50.6% were current smokers (n = 1,758), with 60.7 years (standard deviation 8.8 years).
- Lung-RADS 4 was observed in 233 (6.7%) patients.
- Biopsy was indicated by minimally invasive methods in 122 patients (3.5%).
- Two patients who demonstrated false-negative biopsies and lung cancer were diagnosed in follow-up.
- Diagnosis of lung cancer was observed in 74 patients (prevalence rate of 2.1%), with 52 (70.3%) in stage I or II.
- Granulomatous disease was found in 20 patients.
There were no statistical differences in the incidence of lung cancer, biopsies, granulomatous disease, and Lung-RADS 4 nodules between public and private patients.
CONCLUSION
There are still many challenges and obstacles in the implementation of LCS in developing countries;
The authors hope that it could stimulate the creation of organized screening programs in regions still endemic for tuberculosis and other granulomatous diseases.
however, our multi-institutional data were possible to obtain satisfactory results in these scenarios and to achieve similar results to the main international studies.
Granulomatous diseases did not increase the number of lung biopsies.
The authors hope that it could stimulate the creation of organized screening programs in regions still endemic for tuberculosis and other granulomatous diseases.
There are still many challenges and obstacles in the implementation of LCS in developing countries;
The authors hope that it could stimulate the creation of organized screening programs in regions still endemic for tuberculosis and other granulomatous diseases.

Context
The reality of lung cancer in Brazil is similar to that found worldwide.
The disease’s high prevalence and lethality are related to cigarette use and late diagnosis, with more than 90% of cases in an advanced stage.1–3
Key Objective
To confirm lung cancer screening (LCS) feasibility in different regions of Brazil.
Knowledge Generated
The Second Brazilian Early Lung Cancer Screening Trial is the first multi-institutional LCS study in Brazil with 3,819 enrolled participants.
It achieved similar results of the first Brazilian Early Lung Cancer Screening Trial and other international studies.
The authors reported at JCO Global Oncology analogous outcomes of the main international studies confirming 2.1% prevalence of lung cancer.
Lung cancer was diagnosed in 74 of 122 suspicious nodules (3.5% biopsy rate). Granulomatous diseases did not increase the number of biopsies.
Relevance
There are innumerous challenges for LCS in Brazil; however, it was possible to obtain satisfactory results in all regional scenarios.
This work may stimulate the creation of LCS programs in Latin America, which are also important to other endemic regions for granulomatous diseases.
Introduction
Lung cancer screening (LCS) with low-dose computed tomography (LDCT) is becoming the standard worldwide.
The literature has consistently presented scientific evidence proving the efficiency of detecting lung nodules and tumors, including early-stage cancer, through randomized and high-quality studies. The results of the Early Lung Cancer Action Program (ELCAP) study showed superior performance of LDCT, compared with X-ray, in detecting small lesions.4With these results, other screening programs were initiated in Europe and the United States. The National Cancer Institute conducted a randomized study to determine whether screening for lung cancer using LDCT could affect lung cancer mortality-the National Lung Screening Trial (NLST).5After 8 years of follow-up, the findings indicated a significant 20% reduction in the number of deaths from lung cancer and other causes. More recently, the European study Nelson achieved even more significant results in reducing mortality, between 40% and 60% among men and women, respectively.6
Currently, different international medical entities have recommended LCS for high-risk populations, including the National Comprehensive Cancer Network (NCCN) and the US Preventive Services Task Force (USPSTF).7,8
However, the literature is scarce in countries with a high incidence of granulomatous disease. Brazil is one of the top 30 nations globally with a high incidence of tuberculosis (TB) disease.
According to Brazilian data, there was an estimated TB incidence rate of 46 per 100,000 population in 2019. In countries such as ours, it is important to achieve an equilibrium between an approach to detect and resecting malignant lesions at initial presentation and minimize resection of benign lesions.
Since the NLST results in 2011, efforts have been made to implement screening programs in Brazil.
The First Brazilian Early Lung Cancer Screening Trial (BRELT1) in Brazil was a single-institution study that included only 790 patients.9This study demonstrated that it is possible to implement LDCT screening, performing a similar number of biopsies compared with other international studies such as NLST,5Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON),6Continuous Observation of Smoking Subjects.10The Pittsburgh Lung Screening Study,11and others.12,13BRELT1 also demonstrated a higher percentage of patients identified with an early-stage lung cancer diagnosis, rather than a larger proportion of patients with advanced diseases, typically reported in this country.9
The current study is a multicenter retrospective report involving several Brazilian institutions that have been carrying out LCS in Brazil.
The study was performed to determine whether the preliminary results of the BRELT1 would be maintained when patients from institutions from different regions of Brazil were included.
This is a retrospective, cohort study, with follow-up of individuals with a history of heavy smoking, who participated in LCS programs between December 2013 and January 2021 in six Brazilian institutions.
This study was approved by the institutional review boards of the screening centers. Informed consent was not required.
The individuals were recruited according to the screening criteria recommended by the International Early Lung Cancer Action Program (I-ELCAP)14and NLST5and ratified in the guidelines of the NCCN7and USPSTF.8
For this study, to improve the homogeneity of the sample, only the outcomes of current smokers or exsmokers for a maximum of 15 years, with a smoking history of 30 pack-years or more, age between 54 and 80 years, with no lung cancer suggestive symptoms were analyzed.
These patients underwent at least one LDCT in a LCS program, and the results from this initial LDCT were evaluated. All centers were compared between the incidence of cancer, Lung-RADS 4 nodules, and granulomatous disease.
For computed tomography (CT) screening, low-dose 16-multidetector or 64-multidetector CT systems were used to acquire isotropic volume data, without administration of contrast medium.
The maximum slice thickness acceptable was 1.25 mm. The radiation dose ranges between 120 and 140 kVp and 40 and 80 mAs. The acquisition variables were chosen to reduce exposure to an average effective dose of 1.5 mSv. In all participating centers, LDCT examinations were evaluated by a chest radiologist. All LDCT examinations were analyzed using the criteria of the American College of Radiology (ACR), aiming to standardize the recommendations and management reports of the LCS programs (Lung-RADS).15LDCT performed before despite the publication of Lung-RADS was reclassified by the chest radiologists of the participating centers.
LDCT classified as Lung-RADS 1 and 2 was reassessed 1 year after the first one.
Cases with Lung-RADS 3 were reassessed in 3–6 months. The LDCT categorized as Lung-RADS 4 (4A, 4B, or 4X) was considered suspect and analyzed by local multidisciplinary committees.
The examinations classified as Lung-RADS 3 and 4 were also evaluated for the characteristic features of granulomatous disease, considering previous infection findings (eg, bronchiectasis, the number of calcified lymph nodes, calcified mediastinal lymph nodes, and fibrotic scar in the upper lobes).
Cases suspicious for granulomatous disease were followed clinically. Patients who underwent biopsy were evaluated to define the prevalence of granulomatous disease in this group.
To complement the assessment of individuals with tests classified as Lung-RADS 4, positron emission tomography-CT was used, when indicated, and transthoracic needle biopsy, bronchoscopy, or surgery for histopathologic confirmation was performed.
If all minimally invasive biopsies were negative, a follow-up of 2 years was required for benign disease diagnosis. Diagnosis and staging of lung cancer, diagnosis of other pathologies related to smoking, and diagnosis of granulomatous diseases, with emphasis on TB, were considered as outcome variables. The first examination cycle data were used to establish the prevalence of lung cancer cases and their clinical and pathologic staging in the recruited population.
Patients with lung cancer diagnoses were referred for treatment according to established best medical practice.
Cases with suspicion or diagnosis of other tobacco-related diseases or infectious diseases were similarly referred for treatment and follow-up.
Statistical analyses were performed using IBM SPSS Statistics Software (version 21; IBM Corp, Armonk, NY).
Discrete variables were presented as the number of cases unless otherwise specified. Continuous data were presented as mean ± standard deviation (SD; minimum-maximum). In the descriptive analysis, categorical variables were expressed in absolute number and percentage and continuous data were expressed as mean (SD). A chi-square test was used to test the consistency of incidences. A P-value of < .05 was defined as statistically significant for all results.
Between December 2013 and February 2020, 4,365 individuals were screened at six institutions located in the south, southeast, and northeast regions of Brazil.
For homogeneous analysis of the data, all individuals (n = 895) subjected to tomography, but who did not meet the inclusion criteria of the I-ELCAP, NLST, NCCN, and USPSTF, were excluded. Most of the patients were included from private practice (3,069 patients). There were no statistical differences in the incidence of lung cancer, biopsies, granulomatous disease, and Lung-RADS 4 nodules between public and private patients.
Therefore, 3,470 individuals were included in this analysis, of which 59.8% were male (n = 2,074) and 50.6% were current smokers (n = 1,758), with a mean age of 60.7 years (SD 8.8 years).
From initial LDCT, Lung-RADS 4 was observed in 218 (6.3%) patients. A biopsy was performed by minimally invasive methods (CT-guided biopsies in 82 and bronchoscopy-guided biopsy in 40 patients) in 109 patients (3.14%). Diagnosis of lung cancer was observed in 73 patients (prevalence rate of 2.1%), with 52 (70.3%) in stage I or II. Granulomatous disease was found in 20 patients, and 26 patients had stable CT follow-up diagnoses of benign disease. Two patients who demonstrated false-negative biopsies and lung cancer were diagnosed in follow-up ( Fig 1).
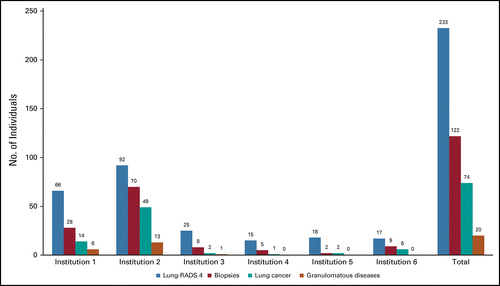
The incidence of lung cancer, biopsies, granulomatous disease, and Lung-RADS 4 nodules by the center is described in Table 1.
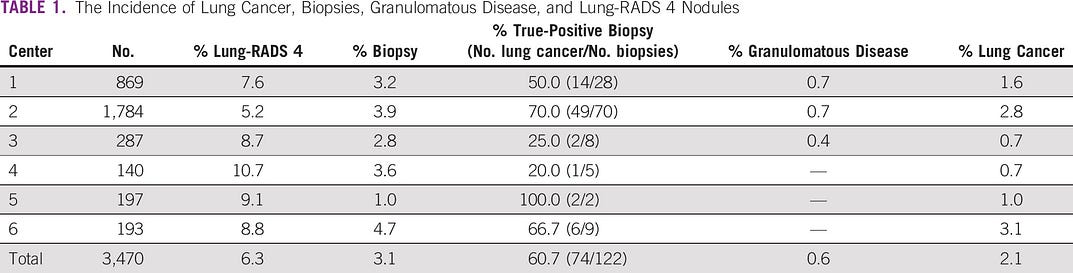
The Incidence of Lung Cancer, Biopsies, Granulomatous Disease, and Lung-RADS 4 Nodules
The treatment was established according to the rules recommended by the NCCN, Ministry of Health of Brazil, and the surgical approach was determined in all centers using minimally invasive techniques.
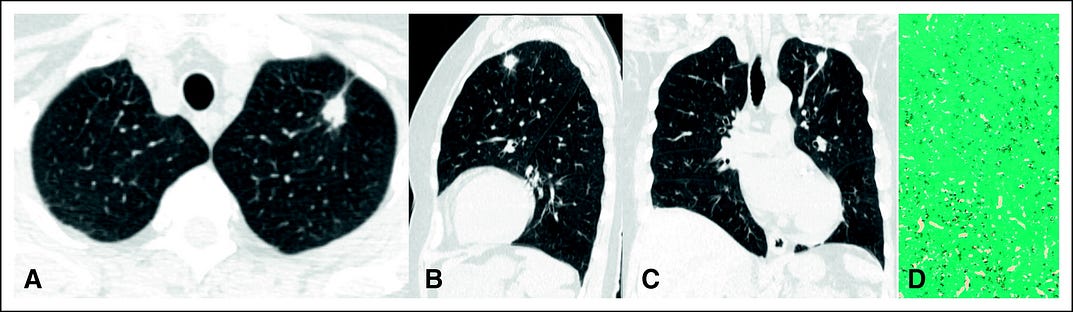
Figure 2 shows LDCT images from a 65-year-old man from south of Brazil who participated in the LCS program. His medical history included a 60-pack-year smoking and previous treatment of pleural TB. The LDCT showed a spiculated pulmonary nodule classified as Lung-RADS 4Bx. CT images with coronal and sagittal reconstruction demonstrated the same findings. CT-guided biopsy specimens contained predominantly noncaseating granulomas and intracellular and extracellular fungal elements compatible with histoplasmosis (Grocott, ×400).
In the present Brazilian multi-institutional study, the prevalence of lung cancer was similar to the single-institution BRELT1 study, raising great expectations in LCS programs in a country with a high incidence of granulomatous disease.
Until 2004, there was no consistent reduction in lung cancer mortality in Brazil.16
More recently, this scenario is changing, and it is possible to identify some reduction in the Brazilian lung cancer death rate in specific settings and subpopulations, which might reflect a downward trend in smoking prevalence associated with improvements in the Brazilian health care system.17–19
Latterly, in Brazil, the availability of physicians and chest CT examinations has expanded.20
However, one of the basic and important barriers to LCS is the number of CT scanners in the public system, which is responsible for the health care of approximately 80% of the Brazilian population.
The private system has six times more CT scanners, with numbers comparable with those found in high-income countries.
However, one of the basic and important barriers to LCS is the number of CT scanners in the public system, which is responsible for the health care of approximately 80% of the Brazilian population.
A recent study by Ponte et al,3which evaluated the relationship between the availability of chest CT examinations and lung cancer death rate in Brazil, has shown that the increasing availability of chest CT examinations was associated with the reducing lung cancer death rate in Brazil, despite no strict protocol for screening lung cancer.
There is sufficient evidence in the literature to indicate population screening for lung cancer; however, its implementation has numerous challenges.
Given the expansion of LCS programs worldwide and technological advances, efforts were made in Brazil to implement screening in different regions, most of them still endemic for granulomatous diseases.
The Brazilian health system provides universal access to health and consists of public and private systems.
The greatest challenge of modern medicine, with the growing demand for high-cost technology, is finding ways to benefit many persons without exponentially increasing costs.
- This challenge embraces the need to integrate the private system’s activities with the public system, generating demand and access in an organized and structured way to various technologies already available in some regions.
- Another possible path to reduce costs in the health system is the verticalized organization, where all the health care, including prevention, diagnosis, and treatment, is organized as a single company. This kind of management favors screening and prevention programs because of the reduction of the high costs of treating advanced diseases.
The main limitation of this study is that it is retrospective and from multiple institutions. This limits the number and consistency of variables collected among sites.
There might have also been small differences in the selection of patients for screening. However, when focusing on patients using the criteria defined in the NLST study, biopsies were indicated in 3.14% of the screened population, which is very close to the values of BRELT1 (3.1%),9The Lung Screening Study (3.6%),21ELCAP (2.8%),4and NLST(2,8%).5In addition, the prevalence of lung cancer in this study (2.1) remained quite similar to the other international studies such as ELCAP (2.7)4, Lung Screening Study (1.9),21NELSON (2.6),6and Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE; 2.2).22
It is a well-known fact that in Brazil, the majority of lung cancer cases are diagnosed in advanced stages.2,17
The present study demonstrated a significant stage shift, with 70% of cancers diagnosed as early stage.
In Brazil, surgical treatment represents a much lower cost than other treatments with chemotherapy, radiotherapy, or agents such as immunotherapy or target therapy.
BRELT2 diagnosed more early-stage than advanced-stage cases with low-dose chest tomography.
Therefore, there is the possibility of cost reduction in the treatment of lung cancer in Brazil; however, we recognize that larger population-based screening programs and prospective studies are required to confirm this hypothesis.
BRELT2 diagnosed more early-stage than advanced-stage cases with low-dose chest tomography.
Therefore, there is the possibility of cost reduction in the treatment of lung cancer in Brazil; however, we recognize that larger population-based screening programs and prospective studies are required to confirm this hypothesis.
Evidence of the effectiveness of LCS is clear worldwide.
In Brazil, difficulties in its implementation related to
- the organization of the health system,
- access to CT scans and treatment methods,
- and cultural acceptance of the method slow down the process.
This first multi-institutional series was carried out independently in different regions of Brazil.
Fortunately, it was possible to achieve similar results to the main international studies. Granulomatous diseases did not increase the number of lung biopsies.
In conclusion, there are still many challenges and obstacles in the implementation of LCS in developing countries; however, our multi-institutional data were possible to obtain satisfactory results in these scenarios.
From the data presented in this study, the authors hope that it could stimulate the creation of organized screening programs in Brazil, Latin America, and other regions still endemic for TB and other granulomatous diseases.
© 2022 by American Society of Clinical Oncology

References and additional information
See the original publication
About the authors and affiliations
Borges da Silva Teles, MD, PhD3; Rodrigo Caruso Chate, MD, PhD3; Gilberto Szarf, , MD, PhD3; Marcos Duarte Guimarães, MD, PhD4; Jefferson Luiz Gross, MD, PhD4; Paula Nicole Vieira Pinto Barbosa, MD, PhD4; Rodrigo Sampaio Chiarantano, MD, PhD5; Rui Manuel Reis, MD, PhD5; Edmundo Carvalho Mauad, MD, PhD5; Mario Ghefter, MD3,6; Petrucio Sarmento, , MD, PhD6; Raphael Pereira, MD, PhD7; José Rocha, MD, PhD7; Marcel Lima Albuquerque, , MD, PhD7; André Miotto, , MD, PhD8; Daniela Cristina Almeida Dias, MD, PhD8; Juliana P. Franceschini, PhD6; Hiran C. Fernando, MD, PhD9; and Ricardo Sales dos Santos, MD, PhD3,10
1Santa Casa de Misericórdia de Porto Alegre, PAVILHÃO PEREIRA FILHO, Porto Alegre, RS, Brazil
2Department of Radiology, University of Florida, Gainesville, FL.
3Hospital Israelita Albert Einstein, São Paulo, SP, Brazil
4AC Camargo Cancer Center, São Paulo, SP, Brazil
5Barretos Cancer Hospital, Cidade Barretos, SP, Brazil
6ProPulmão Program São Paulo, SP, Brazil
7Cardiopulmonar Hospital, Salvador, BA, Brazil
8Sancta Maggiore Hospital, São Paulo, SP, Brazil
9Allegheny General Hospital, Pittsburgh, PA
10SENAI CIMATEC University Center, Salvador, BA, Brazil
Originally published at https://ascopubs.org.