the health strategist
institute for continuous transformation
in health and tech
Joaquim Cardoso MSc
Founder and Chief Researcher & Editor
March 16, 2023
Quick Takes
- Digital health solutions are rapidly growing in number and have the potential to improve health access, outcomes, and equity, as well as optimize operational efficiency and costs for organizations
- Defining the evidence requirements for digital health solutions is complex and is highly dependent on the intended purpose of the solution itself
- Bridging the evidence gap that exists today and generating robust evidence in a timely and cost-effective manner will empower healthcare transformation
DEEP DIVE

Closing the evidence gap for digital health solutions
HealthcareTransformers
Dr. Matthew S. Prime, Saira Ghafur
15 March 2023

Digital health solutions are rapidly growing and have the potential to revolutionize healthcare.
This growth is reflected by the rising number of companies emerging in the sector and increasing amounts of investment in digital health over the last decade.1,2 Notably, the unique challenges generated by COVID-19 abruptly catapulted the positioning of using digital health solutions from an interesting potential opportunity to an immediate necessity.3
A wide variety of technologies — medical apps, clinical decision software, electronic health records, telehealth, health information technology, wearables devices, artificial intelligence, and machine learning — can be considered digital health solutions.
These solutions have the potential to improve health access, outcomes, equity, as well as operational efficiency and costs for healthcare organizations.
However, key challenges remain:
- How can healthcare providers or users of digital health solutions determine whether they deliver the value they claim, and
- What evidence should companies providing digital health solutions generate to prove it?

The complexity of defining evidence needed for digital health solutions
Given the spectrum of digital tools and technologies created for the healthcare market, there can be no single standard for what evidence is required for digital health solutions as a group.
The evidence required is highly dependent on the intended purpose of the solution itself.
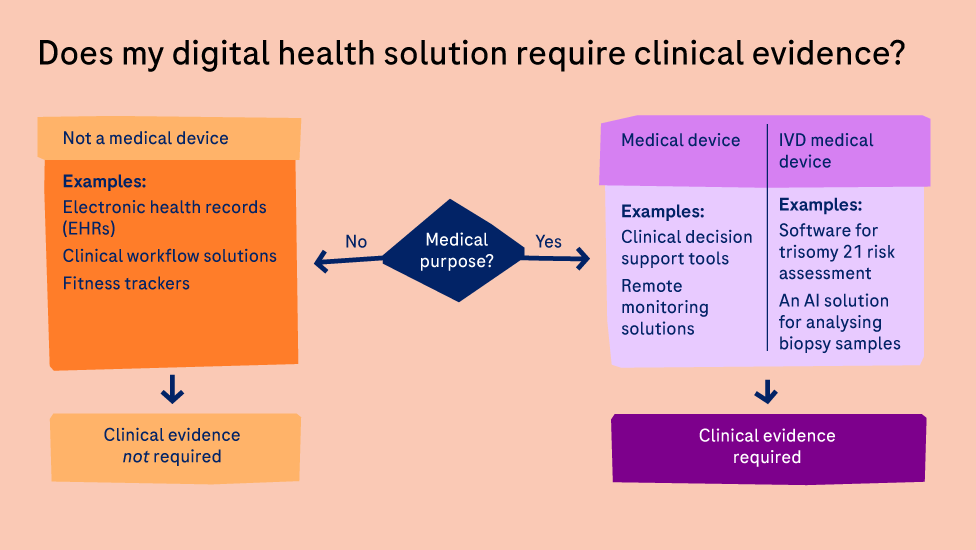
Digital health solutions with a medical purpose qualify as a medical device (also known as Software as a Medical Device (SaMD) in the US, Medical Device Software (MDSW) in the EU, or digital therapeutic).
These solutions need regulatory approval to enter the market and require clinical evidence to show their safety and efficacy.
The evidence required for these solutions include:4
- Clinical association: Does the solution solve a real problem?
- Analytical performance: Does the solution perform its calculations accurately and reliably?
- Clinical performance: Does it produce clinically relevant data in its target population and environment?
This is not the case for digital health solutions with no medical purpose and therefore not considered a medical device such as electronic health records or clinical workflow solutions.
To demonstrate the value of these solutions, as well as the additional benefits of digital solutions with a medical purpose, other evidence is of importance to allow various stakeholders to make decisions about their implementation.
These include:4
- Clinical value: Does the solution have a measurable impact on clinical outcomes (peer-reviewed evidence)?
- Financial value: Does the solution show benefit as shown by health economics and outcomes research (if it directly impacts patient care), or show an enterprise benefit via a financial model?
- Operational value: Does the solution provide operational efficiencies?
- Experience value: Does qualitative research demonstrate its usability and adoption potential?
What are the different types of evidence needed for digital health solutions?4
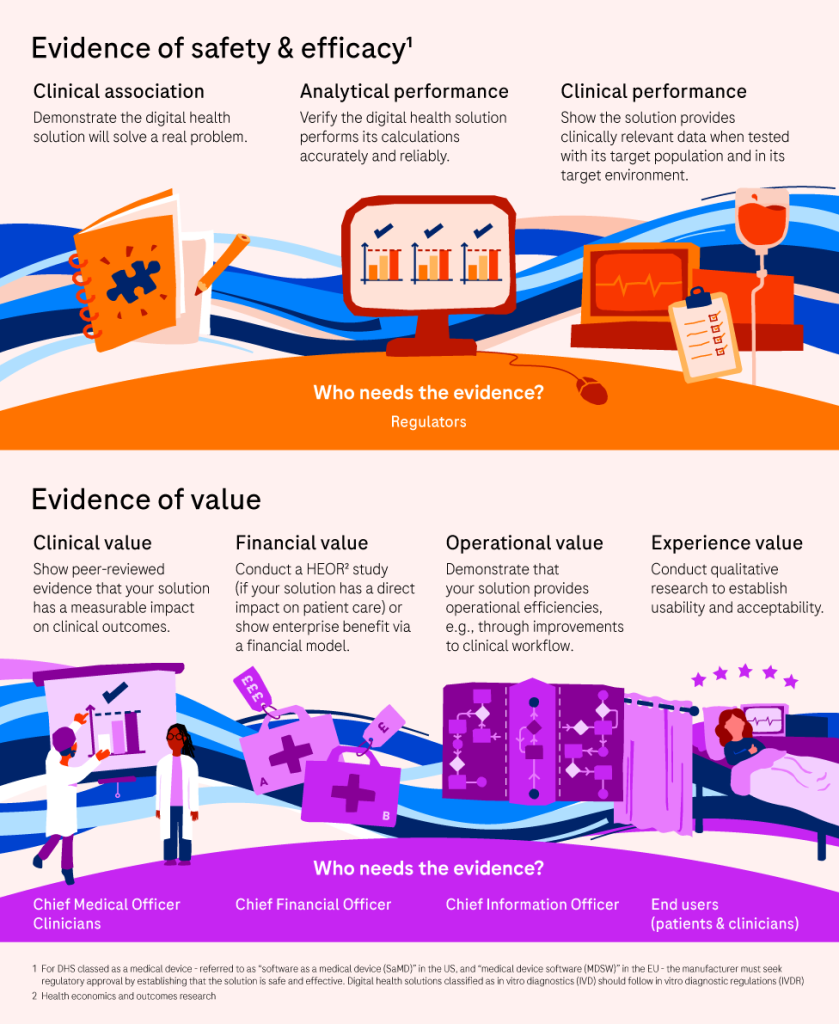
Generating evidence for digital health solutions
Breaking new ground by investigating pragmatic approaches to demonstrate the value of digital health solutions such as clinical simulation, this white paper looks ahead to promote the delivery of impactful, evidence-based solutions to clinicians and patients.

Closing the existing gaps in evidence
Regardless of the type of evidence required, today many digital health solutions land on the market without generating appropriate data.
One study analyzed all 224 US-based digital health companies focused on prevention, diagnosis, or treatment of disease that raised at least one round of venture funding of $USD 2 million or more between 2011–2020.
This study revealed that 44% of the digital health companies had no regulatory filings nor clinical trials published. 5
There are various reasons why this may be the case. Many solutions entering healthcare today belong to the software developers and product experts of the tech world, rather than traditional health stakeholders.
While these new players possess a wealth of expertise, they are not necessarily familiar with established clinical research principles and practices that are the backbone of the traditional biopharmaceutical industry.
In addition, the emergency situation that COVID-19 caused for healthcare systems may have led to unchallenged tools on the market.6
Evidence generation can also be very expensive, and considering that in most markets reimbursement pathways that reward good evidence do not exist, there is often too little incentive for manufacturers to make this investment.
Innovators may also not have access to the data that would help generate strong evidence for their solutions, and may not have the capacity to gather and store this in a secure manner.
Furthermore, traditional research methodologies, such as randomized control trials — the gold standard for assessing drugs and other interventions — are ill-suited to many digital health solutions that share the same therapeutic aim.
Furthermore, traditional research methodologies, such as randomized control trials — the gold standard for assessing drugs and other interventions — are ill-suited to many digital health solutions that share the same therapeutic aim.
This lack of rigorous evidence can result in the use of ineffective tools in the marketplace, and, perhaps even more worryingly, compromised patient safety.7,8
Furthermore, it dampens enthusiasm for and uptake of future digital solutions.
In order for technology to truly transform healthcare, the industry will need to find ways to bridge the evidence gap that exists today for digital health solutions and generate robust evidence in a timely and cost-effective manner.
Fortunately, there are several innovative approaches and new methods that are already being used to help generate the quality evidence needed to demonstrate the true benefits of digital health.
Regulators and payors are starting to embrace and encourage these new and pragmatic approaches, including simulation-based methods and the use of Real World Evidence at the appropriate stages of the development cycle of products.
Additionally, clear reimbursement pathways that reward innovators generating strong evidence are also being formally established in many countries.
Helping to reduce the barriers faced by digital health innovators today will contribute to a stronger global digital health ecosystem, where safer and more effective digital solutions will reach patients and clinicians more efficiently.
References
See the original publication