Health Transformation Institute (HTI)
Value Based Care, Proactive Health, and Digital Health
in the age of Consumer Health
Joaquim Cardoso MSc*
Chief Researcher, Editor and Senior Advisor
December 8, 2022
MSc* from London Business School
MIT Sloan Masters Program (European version)
EXECUTIVE SUMMARY
What is the importance of genomic testing in cancer care?
- Genomic analysis of tumor tissue and circulating tumor DNA is an increasingly important component of cancer care; …
- … such testing can be used to assess cancer risk, inform prognosis, and detect disease recurrence after treatment completion, for example.
- The FDA approach was first used in 1998 — when trastuzumab was paired with an immunohistochemical test that measured expression of human epidermal growth factor receptor 2 (HER2, also known as ERBB2) in breast cancer tissue to determine the likelihood that a tumor would respond to treatment.
- Since then, the use of molecularly targeted therapies and companion diagnostic tests has rapidly expanded.
What is the current situation?
- As of June 2022, the FDA had cleared or approved companion diagnostic tests for approximately 27 molecular biomarkers that guide therapy selection for more than 40 FDA-approved drugs indicated in the treatment of 16 tumor types.
- Five molecular alterations — high microsatellite instability, high tumor mutation burden, NTRK fusions, BRAF V600E mutations, and RET fusions — have been linked to treatments that are approved for use regardless of tumor histologic type or site of origin.
What are the opportunities for NGS (Next Generation Sequencing)?
- Next-generation sequencing (NGS) tests, which reveal the mutational status of hundreds of genes in a single test run, are particularly useful to identify various genomic alterations in tumors that have the same histologic diagnosis.
What is the gap?
- Medical professional societies have played an important role in guiding the appropriate use of tumor genomic testing.
- Evidence from physician surveys and analyses of claims and electronic health record (EHR) data, however, indicate underutilization of tumor genomic testing.
- These findings are in keeping with those of other studies conducted in the United States and internationally that documented molecular-testing rates for NSCLC as low as 20%, depending on the geographic region, practice setting, period in which the study was conducted, and prevailing recommendations for molecular testing at the time.
What are the causes?
- A 2017 survey conducted by the NCI identified various provider- and organization-level factors that may contribute to undertesting.
- Oncologists cited difficulty in obtaining sufficient tissue for testing, insufficient time to order or review tests, and (less often) lack of expert personnel to assist in test interpretation as reasons for not ordering NGS tests. These factors were most often cited by oncologists practicing in rural communities or in solo practices.
Analyses of the survey also found that oncologists with training in genomics or access to a molecular tumor board were more likely than other oncologists to use NGS tests.
- Many factors have been identified that, taken together, suggest poorly coordinated efforts to implement precision oncology care.
What are the recommendations?
- implementing practice-wide workflows that automatically trigger NGS testing for patients with advanced solid tumors,
- make use of molecular tumor boards to interpret results, and provide administrative assistance for clinical-trial matching and obtaining prior authorization are key to successful implementation of precision-medicine programs.4,5
- Building tools into EHRs that assess a practice’s performance against established guidelines for tumor genomic testing is also essential to identify practice-level gaps in care.
- Capturing patient outcomes in EHRs using structured, common data elements will be important to enable sharing of real-world data that could advance knowledge on how best to use precision cancer medicines.
- Prospective randomized trials, meta-analyses of single-group studies, and real-world registry data support the use of comprehensive tumor genomic testing to identify FDA-approved, guideline-recommended therapies for cancer patients.
What is the debate?
- Critics of tumor genomic testing rightly contend that more evidence is needed to support its use outside clinical trials for identifying potential off-label treatments for patients who have exhausted standard therapies, since few such patients currently obtain meaningful clinical benefits from these treatments, and they are at risk for treatment-related and financial harm.
- But, tumor genomic testing is appropriate to identify clinical-trial options for patients with advanced cancer, however.
What are the requirements?
Ensuring equitable and efficient deployment of precision medicine is a global challenge that requires …
- patient access to validated molecular tests,
- a consistent regulatory and reimbursement environment offering incentives for test development, and
- concerted efforts by practice and health system leaders to develop the multidisciplinary workforce …
- … and information infrastructure necessary to create a precision cancer care delivery system.
Putting these pieces in place would not only close the genomic testing gap in cancer care but could transform outcomes for many patients with cancer.
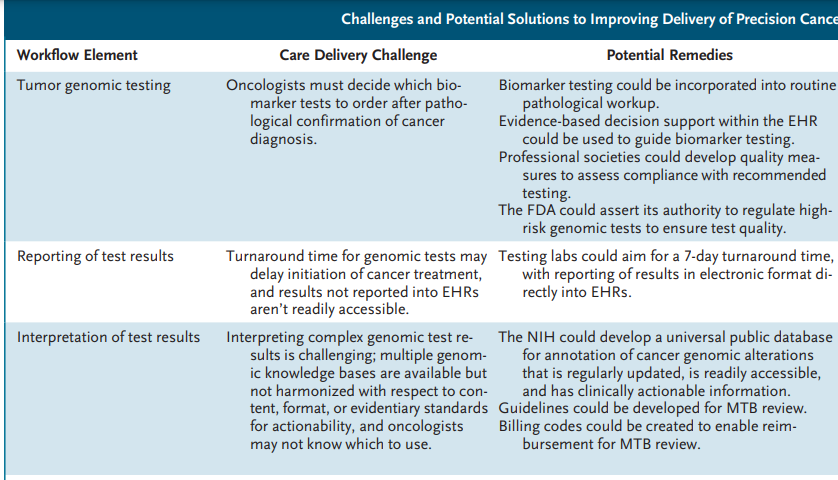
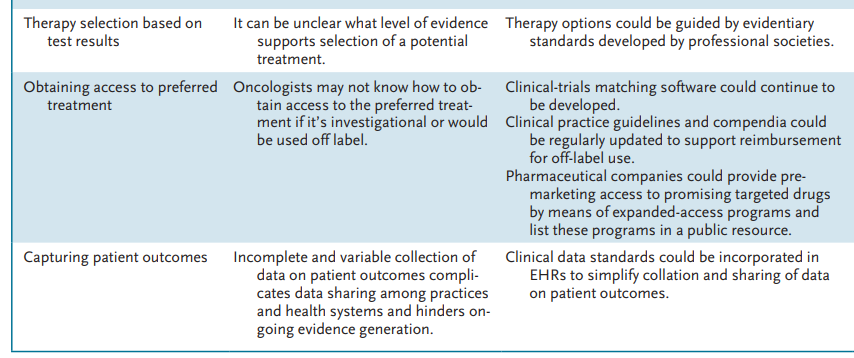
Infographic
Challenges and Potential Solutions to Improving Delivery of Precision Cancer Care.
ORIGINAL PUBLICATION (full version)

Closing the Gap in Cancer Genomic Testing
NEJM
Richard L. Schilsky, M.D., and Dan L. Longo, M.D.
December 8, 2022
Genomic analysis of tumor tissue and circulating tumor DNA is an increasingly important component of cancer care; …
… such testing can be used to assess cancer risk, inform prognosis, and detect disease recurrence after treatment completion, for example.
The most important application of genomic analysis has been in guiding therapy selection with the use of validated diagnostic tests, particularly companion diagnostics.
The Food and Drug Administration (FDA) defines a companion diagnostic test as an in vitro diagnostic device that is essential for the safe and effective use of a corresponding therapeutic product.
Such an approach was first used in 1998, when trastuzumab was paired with an immunohistochemical test that measured expression of human epidermal growth factor receptor 2 (HER2, also known as ERBB2) in breast cancer tissue to determine the likelihood that a tumor would respond to treatment.
Since then, the use of molecularly targeted therapies and companion diagnostic tests has rapidly expanded.
The FDA defines a companion diagnostic test as an in vitro diagnostic device that is essential for the safe and effective use of a corresponding therapeutic product.
Such an approach was first used in 1998, when trastuzumab was paired with an immunohistochemical test that measured expression of human epidermal growth factor receptor 2 (HER2, also known as ERBB2) in breast cancer tissue to determine the likelihood that a tumor would respond to treatment.
As of June 2022, the FDA had cleared or approved companion diagnostic tests for approximately 27 molecular biomarkers that guide therapy selection for more than 40 FDA-approved drugs indicated in the treatment of 16 tumor types.
Five molecular alterations — high microsatellite instability, high tumor mutation burden, NTRK fusions, BRAF V600E mutations, and RET fusions — have been linked to treatments that are approved for use regardless of tumor histologic type or site of origin.
Five molecular alterations — high microsatellite instability, high tumor mutation burden, NTRK fusions, BRAF V600E mutations, and RET fusions — have been linked to treatments that are approved for use regardless of tumor histologic type or site of origin.

Next-generation sequencing (NGS) tests, which reveal the mutational status of hundreds of genes in a single test run, are particularly useful to identify various genomic alterations in tumors that have the same histologic diagnosis.
For example, alterations in as many as a dozen genes in non–small-cell lung cancer (NSCLC) tumors have been linked to FDA-approved treatments.1
NGS tests are widely available from commercial and hospital laboratories, and the FDA has cleared three such tests for tumor molecular profiling.
In 2018, the Centers for Medicare and Medicaid Services finalized a national coverage determination for NGS tumor profiling, concluding that
testing is reasonable and necessary for patients with advanced cancer when the test has been cleared as a companion diagnostic and is performed in a laboratory certified under the Clinical Laboratory Improvement Amendments standards.
testing is reasonable and necessary for patients with advanced cancer when the test has been cleared as a companion diagnostic and is performed in a laboratory certified under the Clinical Laboratory Improvement Amendments standards.

Medical professional societies have played an important role in guiding the appropriate use of tumor genomic testing.
Recently, the European Society for Medical Oncology published recommendations regarding the appropriate clinical use of NGS testing for patients with metastatic cancers, …
… and the American Society of Clinical Oncology (ASCO) recommended that multigene panel testing be performed in patients with advanced solid tumors whenever more than one genomic biomarker has been linked to an approved therapy.
Patient organizations have informed their constituents about and advocated for evidence-based genomic testing, and information for patients is readily available from the National Cancer Institute (NCI), the American Cancer Society, ASCO, and many disease-focused groups.

Evidence from physician surveys and analyses of claims and electronic health record (EHR) data, however, indicate underutilization of tumor genomic testing.
For example, a recent analysis of data from nearly 38,000 patients with stage IV NSCLC diagnosed between 2010 and 2018 revealed that only 22% had molecular test results in their medical record and only 3% were treated with targeted therapy — even though guidelines during this period recommended that all such patients undergo testing for EGFR, ALK, and ROS1 tumor alterations, at a minimum.2
… out of 38,000 patients with stage IV NSCLC diagnosed between 2010 and 2018 , only 22% had molecular test results in their medical record and only 3% were treated with targeted therapy
These findings are in keeping with those of other studies conducted in the United States and internationally that documented molecular-testing rates for NSCLC as low as 20%, depending on the geographic region, practice setting, period in which the study was conducted, and prevailing recommendations for molecular testing at the time.
These findings are in keeping with those of other studies conducted in the United States and internationally that documented molecular-testing rates for NSCLC as low as 20% …
A 2017 survey conducted by the NCI identified various provider- and organization-level factors that may contribute to undertesting.
Oncologists cited difficulty in obtaining sufficient tissue for testing, insufficient time to order or review tests, and (less often) lack of expert personnel to assist in test interpretation as reasons for not ordering NGS tests.3
These factors were most often cited by oncologists practicing in rural communities or in solo practices.
Oncologists cited difficulty in obtaining sufficient tissue for testing, insufficient time to order or review tests, and (less often) lack of expert personnel to assist in test interpretation as reasons for not ordering NGS tests.3
These factors were most often cited by oncologists practicing in rural communities or in solo practices.
When tests were ordered, they were most frequently used to select an FDA-approved therapy or determine eligibility for clinical trials.
Analyses of the survey also found that oncologists with training in genomics or access to a molecular tumor board were more likely than other oncologists to use NGS tests.
Analyses of the survey also found that oncologists with training in genomics or access to a molecular tumor board were more likely than other oncologists to use NGS tests.

What explains the apparent gap between the availability of tumor genomic tests and their utilization?
Many factors have been identified that, taken together, suggest poorly coordinated efforts to implement precision oncology care.
Necessary elements of the cancer precision-medicine workflow include
- comprehensive genomic analysis of tumors,
- interpretation of genomic test results by expert personnel,
- shared decision making with patients about the likelihood that test results will identify effective treatments,
- and employment of administrative services to help patients obtain access to therapies that are identified as potential treatment options (see table).
Each of these processes involves multiple components that must be completed efficiently for care to be delivered within an acceptable time frame.
Such components can include
- identifying or procuring tumor specimens suitable for genomic analysis
- and navigating the uncertainties surrounding clinical-trial eligibility or reimbursement for off-label treatments
… issues that may be particularly challenging for patients who lack the resources to travel to clinical-trial sites or have no insurance or inadequate insurance to cover treatment costs.
Challenges and Potential Solutions to Improving Delivery of Precision Cancer Care.
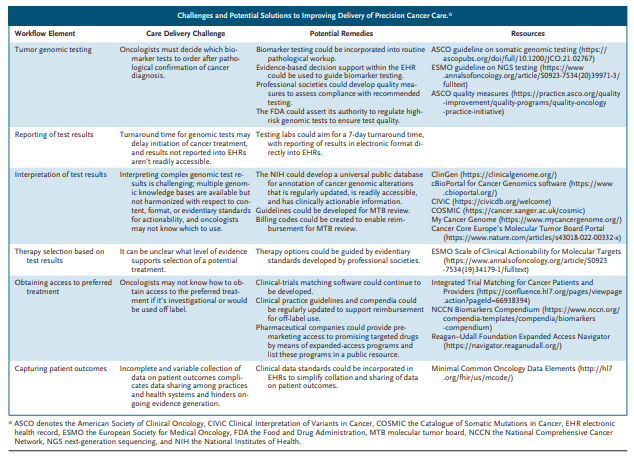
zoom of the large image


Evidence from community practices suggests that :
- implementing practice-wide workflows that automatically trigger NGS testing for patients with advanced solid tumors,
- make use of molecular tumor boards to interpret results, and provide administrative assistance for clinical-trial matching and obtaining prior authorization are key to successful implementation of precision-medicine programs.4,5
Building tools into EHRs that assess a practice’s performance against established guidelines for tumor genomic testing is also essential to identify practice-level gaps in care.
Capturing patient outcomes in EHRs using structured, common data elements will be important to enable sharing of real-world data that could advance knowledge on how best to use precision cancer medicines.
Prospective randomized trials, meta-analyses of single-group studies, and real-world registry data support the use of comprehensive tumor genomic testing to identify FDA-approved, guideline-recommended therapies for cancer patients.
Critics of tumor genomic testing rightly contend that more evidence is needed to support its use outside clinical trials for identifying potential off-label treatments for patients who have exhausted standard therapies, since few such patients currently obtain meaningful clinical benefits from these treatments, and they are at risk for treatment-related and financial harm.
Critics of tumor genomic testing rightly contend that more evidence is needed to support its use outside clinical trials — for identifying potential off-label treatments for patients who have exhausted standard therapies, since few such patients currently obtain meaningful clinical benefits from these treatments, and they are at risk for treatment-related and financial harm.
Tumor genomic testing is appropriate to identify clinical-trial options for patients with advanced cancer, however.
Studies such as the NCI’s Molecular Analysis for Therapy Choice trial and ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) trial show that efficacious treatments can be identified using genomic profiling, even among patients with very advanced disease.
In the TAPUR trial, for which one of us is the principal investigator, about two thirds of screened patients have been assigned to a treatment matched to a genomic alteration in their tumor, with mutations in BRCA1 or BRCA2, CDKN2A, and ERBB2 or ERBB3 each being detected in 10 to 15% of patients with advanced solid tumors.
Among 24 study cohorts (defined according to tumor type, genomic alteration, and study treatment) with available data, there has been a positive signal of drug activity in 16, with disease-control rates between 35% and 70%.
Over time, new molecular tests that can provide information about the tumor epigenome, the transcriptome, the circulating proteome, and the gut microbiome could complement tumor genomic testing and further refine therapy selection for patients.

Ensuring equitable and efficient deployment of precision medicine is a global challenge that requires …
- patient access to validated molecular tests,
- a consistent regulatory and reimbursement environment offering incentives for test development, and
- concerted efforts by practice and health system leaders to develop the multidisciplinary workforce …
- … and information infrastructure necessary to create a precision cancer care delivery system.
Putting these pieces in place would not only close the genomic testing gap in cancer care but could transform outcomes for many patients with cancer.
Ensuring equitable and efficient deployment of precision medicine is a global challenge that requires : (1) patient access to validated molecular tests, (2) a consistent regulatory and reimbursement environment, (3) concerted efforts … to develop the multidisciplinary workforce …and information infrastructure
Originally published at https://www.nejm.org.
About the Authors & Affiliations
Richard L. Schilsky, M.D., and Dan L. Longo, M.D.
From the University of Chicago, Chicago (R.L.S.).