JCO Global Oncology 7:1547–1555.
American Society of Clinical Oncology (ASCO)
Fernando Korkes, MD, PhD1,2; Frederico Timóteo , MD1,2; Suelen Martins, MD3; Matheus Nascimento, MD1; Camila Monteiro , MD1; José H. Santiago, MD1; Willy Baccaglini, MD1,2; Marcel A. Silveira, MD1; Eduardo F. Pedroso, MD1; Marcello M. Gava, PhD1; Prashant Patel , MD4; Phillipe E. Spiess, MD5; and Sidney Glina, MD, PhD1
ABSTRACT
Purpose
- Muscle-invasive bladder cancer (MIBC) is an aggressive disease with a complex treatment.
- In Brazil, as in most developing countries, data are scarce, but mortality seems exceedingly high.
- We have created a centralization program involving a multidisciplinary clinic in a region comprising seven municipalities.
- The aim of this study is to evaluate the impact of a multidisciplinary clinic and a centralization-of-care program (CABEM program) on MIBC treatment in Brazil.
Patients and Methods
- A total of 116 consecutive patients were evaluated.
- In group 1, 58 patients treated for MIBC before establishing a bladder cancer program from 2011 to 2017 were retrospectively evaluated.
- Group 2 represented 58 patients treated for MIBC after the implementation of the CABEM centralization program.
- Age, sex, staging, comorbidity indexes, mortality rates, type of treatment, and perioperative outcomes were compared.
Results
Patients from group 2 versus 1
- were older (68 v 64.2 years, P = .02)
- with a higher body mass index (25.5 v 22.6 kg/m2, P = .017) and
- had more comorbidities according to both age-adjusted Charlson Comorbidity Index (4.2 v 2.8, P = .0007) and
- Isbarn index (60.6 v 43.9, P = .0027).
Radical cystectomy (RC) was the only treatment modality for patients in group 1, whereas in group 2, there were 31 (53%) RC; three (5%) partial cystectomies; seven (12%) trimodal therapies; 13 (22%) palliative chemotherapies; and three (5%) exclusive transurethral resections of the bladder tumor.
No patient in group 1 received neoadjuvant chemotherapy, whereas it was offered to 69% of patients treated with RC.
Ninety-day mortality rates were 34.5% versus 5% for groups 1 versus 2 (P < .002).
One-year mortality was also lower in group 2.
Conclusion
Our data supports that
- a centralization program,
- a structured bladder clinic associated with protocols,
- a multidisciplinary team, and
- inclusion of chemotherapy and radiotherapy treatments
can pleasingly improve outcomes for patients with MIBC.
Ninety-day mortality rates were 34.5% versus 5% for groups 1 versus 2 (P < .002).
One-year mortality was also lower in group 2.
CONTEXT
Key Objective
How a centralization program involving a multidisciplinary clinic was established in Southwest Brazil and resulted in reduction in muscle-invasive bladder cancer treatment–related mortality from 37% to 5% in 2 years.
Knowledge Generated
Without centralization and multidisciplinarity, all patients tend to be treated with radical cystectomy.
After implementing the CABEM program, bladder preservation strategies could also be adopted, and an impressive sevenfold reduction in mortality was observed.
Relevance
Our data support that a centralization program, a structured bladder clinic associated with protocols, a multidisciplinary team, and inclusion of chemotherapy and radiotherapy treatments can pleasingly improve outcomes for patients with muscle-invasive bladder cancer.

LONG VERSION
Introduction
- Bladder cancer (BC) is a common disease, with 430,000 new cases per year worldwide and high lethality, with 165,000 deaths per year.
- In Brazil, 10,640 new BC cases are estimated per year, and
- the Southeastern Region of the country presents the highest BC incidence with 10.54 cases/100,000 inhabitants (4.63 cases/100.000 in Brazil).
Muscle-invasive bladder cancer (MIBC) is an aggressive disease with a complex treatment that involves distinctive medical professionals and disciplines. Mortality rates of the patients treated with radical cystectomy (RC) for MIBC vary widely, with 90-day mortality rates ranging from 0.54% in large-volume academic institutions to more than 13% in the community setting based on North-American data. Several factors have been related to postoperative morbidity and mortality after RC, including patients’ age and comorbidities, hospital and surgeon surgical volume, and urinary diversion. In Brazil, as in most developing countries, data are scarce, but mortality seems exceedingly high.
Several policies have been responsible for reducing the mortality rates in most centers during the past three decades. One of these factors adopted by some developed countries is centralization programs, as reported in England and other countries. In a recent epidemiologic study, we have detected an exceedingly high mortality rate in São Paulo’s public hospitals. There are several public hospitals in this region, and RC was previously performed indistinctively. After noticing this alarming mortality, a centralization program was initiated.
A unified and dedicated BC clinic was founded, denominated the CABEM clinic. CABEM stands for muscle-invasive bladder cancer or câncer de bexiga músculo-invasivo. All patients referred to the centralized CABEM clinic had their cases discussed by a medical board composed of urologists, oncologists, and radiation oncologists. Additional measures were undertaken to obtain better assistance.
This study evaluates the aforementioned BC Centralization Program’s results, comparing MIBC treatment outcomes before and after its implementation.
Inclusion criteria comprised consecutive patients with MIBC treated at the two leading health institutions that belong to the ABC Foundation.
The first cohort of patients evaluated (group 1) comprised a review of the medical registry of 58 consecutive patients treated for MIBC between 2011 and 2017.
The second cohort (group 2) comprised a review of the medical registry of 58 consecutive patients treated after implementing the CABEM multidisciplinary clinic and centralization program (2018–2020).
A unified and dedicated BC clinic was founded (CABEM clinic). The CABEM initiative was advertised to urologists and clinical oncologists in the region, aiming to receive every patient in the ABC region’s public health system with MIBC. All patients referred to the centralized CABEM clinic had their cases discussed by a medical board composed of urologists, oncologists, and radiation oncologists. Additional measures were undertaken to obtain better assistance. The pneumology clinic received all patients interested in quitting smoking. According to Patient Blood Management’s protocol, anemic patients were treated, aiming to reduce the demand for blood transfusion. Furthermore, patients under nutritional risk or malnourished had nutrition supplements prescribed. A unified team operated all patients, and fast-track or enhanced recovery after surgery protocols were routinely adopted. The CABEM initiative was a project aiming to reduce the high mortality rates after observed RC in the ABC region. All patients were operated at the hospital where they were originally treated. All surgeries were performed on the same days of the week, and two members of the CABEM team went to the hospitals to conduce the surgeries and the postoperative care. As the good results of the programs were obtained, they served as motivation for further referrals.
The primary outcome assessed was the mortality rate. Demographic data and treatment details were evaluated ( Table 1). Comorbidities were evaluated according to the American Society of Anesthesiology (ASA) score, the age-adjusted Charlson Comorbidity Index (AA-CCI) adjusted for oncologic cases, and the Isbarn index. Complications were reported according to the Clavien-Dindo Classification. These data were obtained from medical files. Nutritional status was accessed prospectively through the Mini-Nutritional Assessment Short-Form (MNA-SF) and was only available for cohort 2.
Demographic Characteristics
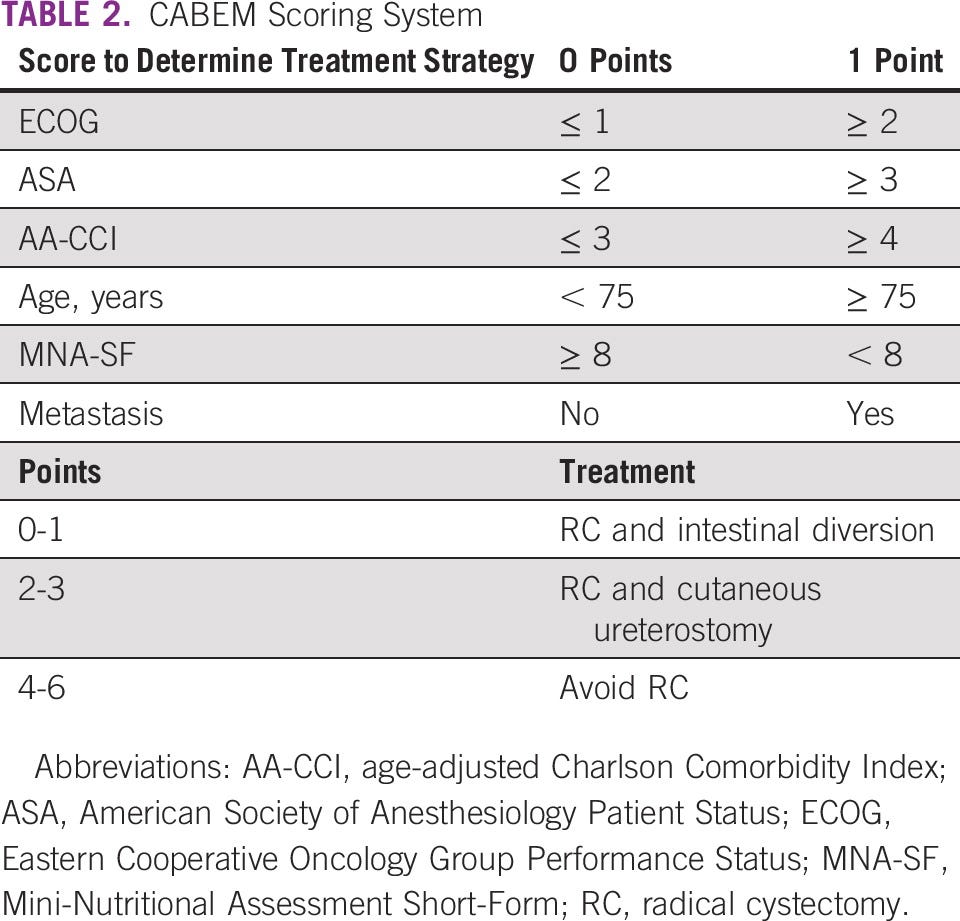
A scoring system was developed to determine whether a patient was eligible to undergo RC and undergo diversion with an intestinal segment. Based on previous studies that determined risk factors for mortality after RC, six factors were carefully evaluated: the Eastern Cooperative Oncology Group Performance Status, the AA-CCI, the ASA, older age (> 75 years), the MNA-SF, and the presence of lymphatic metastasis ( Table 2). For each unfavorable criterion, one point was attributed. If patients had up to two points, RC and an intestinal diversion were adopted. RC could be performed for patients with two to four points, but cutaneous ureterostomy (CU) was chosen as the urinary diversion. For patients with four or more points, bladder preservation alternatives were indicated according to patients’ characteristics (trimodal therapy [TMT], transurethral resections of the bladder tumor, radiotherapy, and/or chemotherapy). Cases were discussed in a multidisciplinary board, including urologists, oncologists, and radio-oncologists. Patients were referred for neoadjuvant chemotherapy (NAC) if they were cisplatin-eligible. NAC was performed with dose-dense MVAC [methotrexate, vinblastine, adryamicin/doxorrubcin, and cisplatin] or gemcitabine and cisplatin. At the beginning of the program, some of the patients were treated with carboplatin and gemcitabine, but this regimen was less used with time.
Surgical Technique Aspects
Trained urologists performed every RC in the study from the two leading public institutions in the ABC region. Procedures were performed either open or laparoscopically.
All CUs were performed with a single stoma. Both ureters were placed side-by-side as a double-barrel or a transureteroureterostomy was completed.
Patients assigned to NAC were given either dd-MVAC or gemcitabine and cisplatin. Dd-MVAC was given as four 14-day cycles as follows: methotrexate (30 mg/m 2of body-surface area) on days 1, 15, 29, and 43; vinblastine (3 mg/m 2) on days 2, 16, 30, and 44; and doxorubicin (30 mg/m 2) and cisplatin (70 mg/m 2) on days 2, 16, 30, and 44. Granulocyte colony-stimulating factor 300mcg (filgrastim) was administered from day 3–14 at each cycle.
Gemcitabine and cisplatin were given as four 21-day cycles as follows:
- Cisplatin (70 mg/m 2) on days 1, 22, 43, and 64; and gemcitabine (1,000 mg/m 2) on days 1, 8, 22, 29, 43, 50, 64, and 71.
- The doses were adjusted if toxic effects occurred.
- For chemoradiotherapy (TMT), cisplatin with fluorouracil was the first choice, followed by single-agent cisplatin or single-agent gemcitabine.
Statistical analysis was performed using SPSS 20.0 (SPSS for Mac OS X, SPSS, Inc, Chicago, IL). Groups were compared with Pearson’s chi-square or Fisher’s test. The Student T-test was used for continuous variables with normal distribution, and the Mann-Whitney U test for non-normal distribution variables. Analysis of variance was performed for multiple comparisons. Overall survival was estimated using the Kaplan-Meier method and log-rank test. Statistical significance was determined at P < .05.
All participants who could be contacted have voluntarily provided informed consent and were aware that they could withdraw consent, as required by our institutional review board. Our study was conducted after institutional review board approval (Protocol Number: 3.853.008).
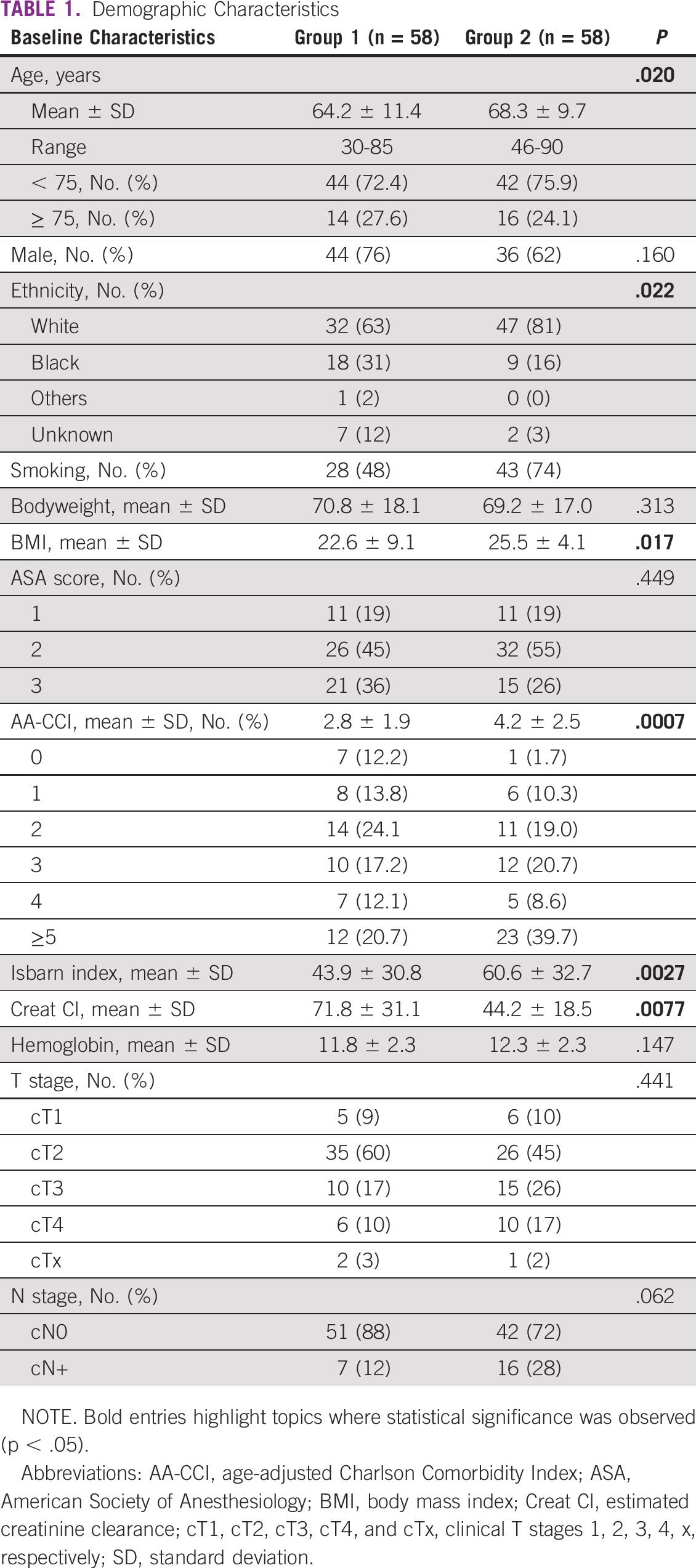
A total of 116 patients were included in the present analysis, 58 in group 1 and 58 in group 2. Patients’ demographics are presented in Table 1. Patients in group 2 versus group 1 were older (68.3 ± 9.7 v 64.2 ± 11.4 years, P = .02) with a higher body mass index (25.5 ± 4.1 v 22.6 ± 9.1, P = .017) and more comorbidities according to both AA-CCI (4.2 ± 2.5 v 2.8 ± 1.9, P = .0007) and Isbarn indices (60.6 ± 32.7 v 43.9 ± 30.8, P = .0027). Ethnicity was also different between both groups. The BC stage was similar between both groups, with many patients with advanced disease, including clinical T stages 3–4 (cT3-T4) and cN+.
All 58 patients in group 1 were treated with RC right after MIBC diagnosis, whereas 53% of group 2 were managed with RC. BC cT1-cT4 for indication of RC were, respectively, in group 1: 9% (five), 60% (35), 17% (10), and 14% (eight), and in group 2: 13% (four), 23% (seven), 32% (10), and 32% (10). Patients had significantly higher clinical stages in group 2 (3 ± 1 v 2 ± 1, P = .007).
When comparing patients in group 1 versus 2 who underwent RC, 90-day mortality was still significantly higher in group 1 (34.5% v 9.6%, P = .01, Appendix Table A1). Patients in group 1 and 2 who underwent RC had similar ages (64.2 ± 11.4 v 65.3 ± 8.4, P = .32), similar sex ratio ( P = .25), similar CCI (2.8 ± 1.9 v 3.4 ± 2.3, P = .09), and similar Isbarn score (43.9 ± 30.8 v 55.5 ± 34.1, P = .052). Patients in group 1 versus 2 had a higher ASA score (2.2 ± 0.7 v 1.9 ± 0.7, P = .04). Ileal conduit was the urinary diversion of choice for 95% of patients from group 1, and CU was significantly more frequent in group 2 versus 1 (63.3% v 0, P < .0001). In group 2, CU was adopted according to the CABEM criteria ( Table 2).
In group 2, three patients (5%) were treated with partial cystectomy after NAC; three (5%) received exclusive transurethral resections of the bladder tumor; seven (12%) received TMT; and 13 (22%) received palliative chemotherapy.
None of the patients from group 1 underwent NAC. In group 2, 23 patients were referred to their respective clinical oncology units for evaluation, with 15 receiving NAC. Considering NAC treatment scheme, seven patients (46%) received gemcitabine plus cisplatin; three (20%) received dd-MVAC; and five (33%) received carboplatin. Pathologic response rates and treatment toxicities are shown in Appendix Table A2.
We have retrospectively calculated the CABEM score for group 1, although MNA-SF was not available. In such a case, 30 of 58 (51.7%) patients scored between three and six points. Twenty-seven patients (46.4%) scored two or three points, and 12 patients (20.7%) scored between four and six points.
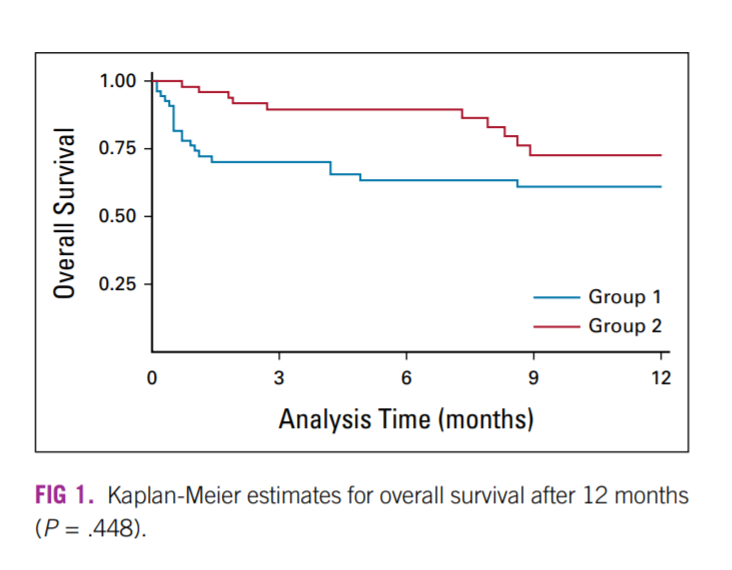
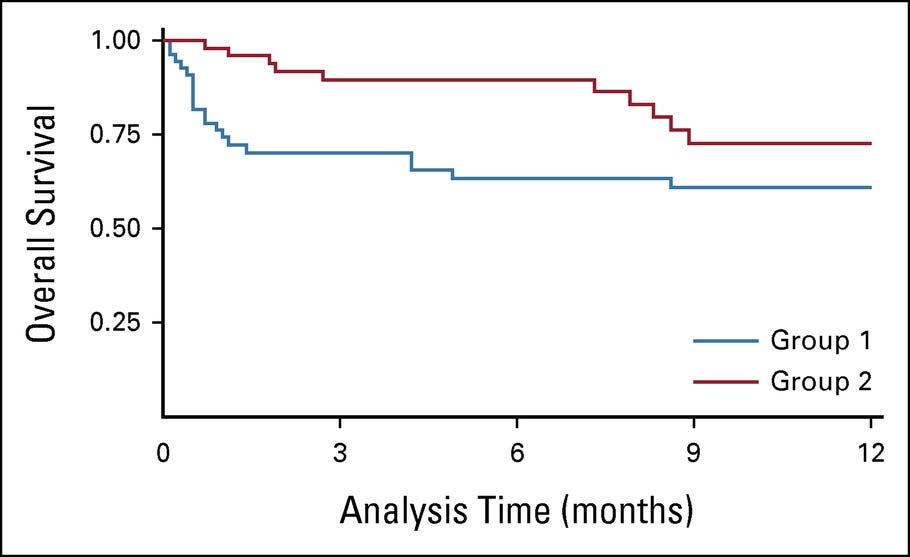
Major complications (Clavien-Dindo 4 and 5) were significantly more common in group 1 (Appendix Table A1). Thirty-day mortality and ninety-day mortality (CD5) were also more common in group 1 (25.9% v 3.4%, P < .0001; and 34.5% v 5.2%, P = .0002, respectively, Appendix Table A1). For patients who underwent RC, the median ± standard deviation (SD) length of hospital stay was shorter in group 2 versus group 1 (5.5 ± 6.7 v 13.5 ± 17.9, P = .0047). All patients were kept at the hospital until fully capable of discharge since there are no hotels or locations for community nursing. Overall survival after 12 months was significantly better for group 2 versus 1 (74% v 57%, P = .448, ).
BC is an aggressive disease. Mortality rates are high if left untreated. By contrast, it frequently affects old, frail, and ill patients. Among urologic surgeries, RC is considered to be associated with the highest complication and mortality rates. Additionally, treatment modalities are complex and also morbid. All these factors combined compound the complexity of the treatment of this disease. We aim to offer our patients current best practices and what might be done worldwide. In such a manner, our program does not seem to be unique. However, the process that we have gone through might bring valuable insights to other centers in developing countries. The improvements that we have obtained were acquired more through processes and protocols than through massive investments. We believe that this study has some meaningful findings, demonstrating that some relatively simple changes can directly affect patient outcomes.
It is well known that RC increases lifetime and is far superior to observation for patients with MIBC. TMT is also efficient in well-selected patients. Even so, only the minority of patients with MIBC have the opportunity to undergo effective curative treatments.
Epidemiologic series demonstrates that in Sweden, less than half of the patients with MIBC have the opportunity to receive a curative treatment with RC. In the United States, < 20% of patients with MIBC undergo RC. In the United States, 76.5% of patients who receive curative treatment for MIBC undergo RC, and 23.5% TMT. Less than 7% of all octogenarians with nonmetastatic MIBC in the United States undergo RC. The majority of patients with MIBC do not receive treatment as recommended by current guidelines. It is imperative to state that although RC is the optimal treatment for MIBC, it comes at a high price. For the more fragile patients, we have to be sure that we are first not harming them. We have to understand that alternative treatments’ results for these frail patients might be better and balance the risks and benefits for each case. We believe that precisely the opposite from what has been reported in the United States was happening in our region. In the United States, only a few patients undergo RC. In group 1, almost every patient was being treated with RC. And that was coming at a high price, with unacceptable mortality rates. It is important to reinforce that there have been progressive improvements in the field of BC treatment during the past 15 years. NAC and TMT have been more applied during the past decade. However, it is still noteworthy the rapid decrease in mortality rate observed. This outcome change seems to be more because of a centralized multidisciplinary approach than to any other factor.
If patients in group 1 were treated according to the CABEM score, in 30 of 58 (51.7%), we would have chosen a different approach. In 27 patients (46.4%), we would have performed an RC without intestinal diversion, and in 12 patients (20.7%), we would have recommended an alternative approach, avoiding RC. It is important to reinforce that MNA-SF was not available for patients in group 1, and therefore these results could be even worse.
Current guidelines recommend RC as a first-line treatment for MIBC, and intestinal segments are used as the preferred method of urinary diversion. Classic urinary diversions, such as an orthotopic neobladder, an ileal conduit, or continent reservoirs, are attractive options and bring long-term benefits for most patients. Nevertheless, several authors have demonstrated that urinary diversions are responsible for many postoperative complications after RC. These complications are a concern mainly in the more fragile patient. As seen in our cohort, we receive patients in poor clinical conditions with high comorbidity indexes ( Table 1). AA-CCI of three or more was encountered in 59.5% of our patients (69 of 116).
Additionally, 62.1% of our patients were anemic at the first appointment (72 of 116), and 17.2% (20 of 116) had severe anemia, with hemoglobin lower than 10 g/dL. Moreover, 57% of our patients were under nutritional risk or malnourished. Anemia and low albumin have both been previously associated with higher complication rates and higher mortality after RC and intestinal diversion. Anemia was associated with an odds rate of 1.49 of mortality, and low albumin increases the risk of mortality by 1.93–2.33. The tumors we treated were at an advanced stage, and almost half of our patients presented with locally advanced disease (35.3% with cT3-T4 and 19.8% with cN+).
In such a context, we have adopted a strategy to predict not only patients who are frail because of advanced age and comorbidities but also nutritional status. According to the CABEM score obtained, we have decided whether to use an intestinal segment for the urinary diversion ( Table 2, ). All our patients who underwent CU were left with a single stoma, which allowed for very reasonable life quality.
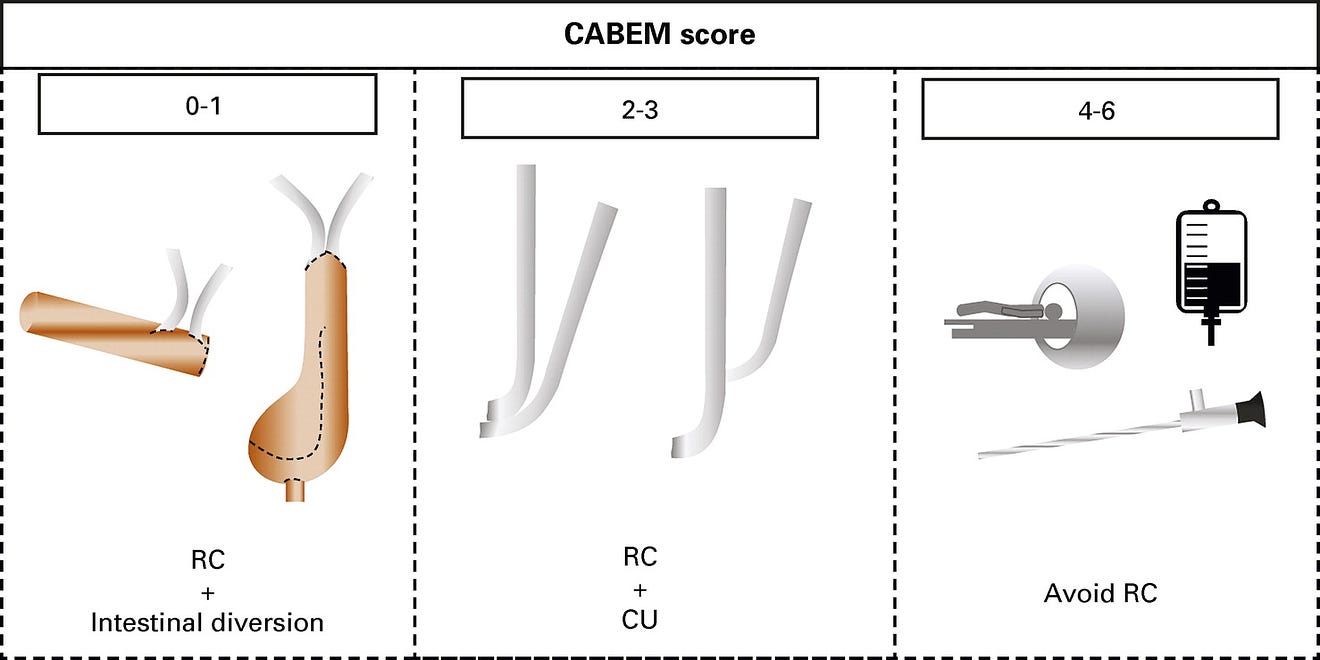
We have composed our scoring system index with factors previously associated with mortality after RC. Age more than 75 years has been associated with an odds ratio (OR) of 1.05–3.04 of mortality. AA-CCI above two has also been associated with increased mortality (OR 1.13–1.7). ASA score above two had an OR of 1.45–5.7, predicting mortality after RC. Eastern Cooperative Oncology Group higher than 1 had an OR of 1.61–2.4. As a surrogate for malnutrition, hypoalbuminemia was also associated with increased mortality after RC, with an OR of 1.9–2.33. We have adopted the MNA-SF to evaluate nutrition because of the simplicity and reliability of this score. Metastatic and locally advanced diseases are also associated with increased mortality after RC.
In developing countries like Brazil, there is a lack of data about BC treatment. Epidemiologic studies have demonstrated high mortality rates for surgically treated patients. This high mortality is undoubtedly multifactorial. One strategy that some developed countries have adopted with good outcomes is centralization programs. These programs have been associated with better outcomes and reduced mortality. A recent systematic review has observed that institutions that perform at least 10 and preferably > 20 RCs annually have better outcomes. In our experience, centralization has brought numerous benefits. The integration with clinical oncology and radiotherapy has opened possibilities of distinctive and more complex treatment options previously withheld from patients in the ABC region. There was a marked difference in treatment strategies before and after the implementation of the CABEM program. Because of a lack of communication and institutional treatment protocols, patients with MIBC were systematically treated with RC in the years before CABEM. However, after the coordination and centralization effort of CABEM, NAC and bladder preservation therapies have taken part in MIBC treatment armamentarium for these patients.
Centralization also causes an increment in institutional surgical volume. Although 58 patients were treated within 6 years before CABEM (0.8 patient/month), we have currently treated 82 patients within 27 months after implementing the CABEM Clinic (3.1 patient/month). It represents a fourfold increase in the number of patients treated. This increase in the number of patients treated is associated with better outcomes. In the United Kingdom, performing even one extra case per center brought a statistically significant benefit. Large-volume centers tend to have up to three times lower mortality rates after RC. In our particular case, as our program becomes well known, we now receive not only local patients, but also patients from other regions and other states of the country.
Another important topic was the strategy adopted to allow centralization. Our program has relied on engaging medical professionals in referring patients, engaging public administrators and hospital managers. The primary key for success was guaranteeing the facility to refer patients. We have always assured that it should be effortless for urologists and oncologists to refer their patients, and patients should have direct access to our clinic. First consultations were opened to all comers, and as treatments were conducted, reports were sent back to referring physicians.
The factors mentioned above associated with increased mortality served as a basis for thorough strategies to improve RC outcomes. Improving nutrition status with preoperative protein intake and nutritional therapy is related to a reduction in mortality. Fast-track recovery protocols as the enhanced recovery after surgery have also been associated with lower length of hospital stay after RC. In this study, we have observed similar findings. Fast-track principles were adopted in patients from group 2 who underwent RC. Their median ± SD length of hospital stay was shorter than those from patients in group 1 (5.5 ± 6.7 v 13.5 ± 17.9, P = .0047). As previously mentioned, all patients were kept in the hospital until fully capable of discharge since there are no hotels or community nursing locations.
Athough the median hemoglobin level at the patients’ admission was similar ( Table 1), blood transfusions were less required in group 2 versus group 1 (mean ± SD of 0.4 ± 0.9 v 1.8 ± 2.3, P = .0021, Appendix Table A1). The adoption of the patient blood management protocol and fast-track protocols might have played a role in this improvement observed.
Major complications (CD4) were more common in group 1 versus 2 (29% v 7%, P = .0038). Although surgeries in groups 1 and 2 were all performed by skilled surgeons, patient preparation and selection might be the main factors associated with improved outcomes in the latter. As previous epidemiologic studies have demonstrated, complications after cystectomy are associated with an increased risk of mortality. In our series, mortality was significantly higher in group 1 versus 2 (34.5% v 5%, P = .0002).
Overall survival became significantly better after the implementation of our centralization program. The Kaplan-Meier curves demonstrated the significant initial death rate after surgery in group 1. It also confirmed that after 12 months of follow-up, patients in group 2 still had a 14% higher chance of being alive ().
This study’s primary strength is using robust and predefined criteria to change a previously shattering scenario. Current data are presented to overcome an objective outcome, mortality, with reproducible measures. The coordination and centralization process outlined in our study might bring essential insights into MIBC management. A limitation of the study is that groups were not treated with the same approach. But in fact, that was precisely the consequence of our program. Also, although overall mortality after 12 months was evaluated, long-term oncologic outcomes are not mature.
Although centralization programs have already been adopted for several years in developed countries, we believe that our cost-effective and straightforward centralization model can inspire other centers in developing countries with poor outcomes to improve their results. Additionally, it reinforces the importance of a multidisciplinary approach for the treatment of BC. This complex disease cannot be treated exclusively by surgeons and must have clinical oncologists and radiation oncologists participating in the treatment decisions. Including chemotherapy and TMT as treatment options is essential for the development of a well-structured BC clinic.
In conclusion, BC treatment involves multimodality and integrated decisions. Unless attention is driven to improve outcomes, many centers in developing countries might still reproduce the observed poor outcomes. By contrast, centralization programs associated with protocols and a patient-based approach can pleasingly improve outcomes and bring economic benefit to health care in general. Programs relying on multidisciplinary clinics, well-established protocols, and the creation of large-volume BC treatment institutions are an effective measure to improve outcomes, reduce mortality, and bring cost-effective treatment for BC.
© 2021 by American Society of Clinical Oncology
Conception and design: Fernando Korkes, Suelen Martins, Willy Baccaglini, Phillipe E. Spiess
Financial support: Fernando Korkes
Administrative support: Fernando Korkes, José H. Santiago, Marcello M. Gava, Phillipe E. Spiess, Sidney Glina
Provision of study materials or patients: Fernando Korkes
Collection and assembly of data: Fernando Korkes, Frederico Timóteo, Suelen Martins, Camila Monteiro, Willy Baccaglini, Marcel A. Silveira, Eduardo F. Pedroso, Marcello M. Gava
Data analysis and interpretation: Fernando Korkes, Frederico Timóteo, Suelen Martins, Matheus Nascimento, José H. Santiago, Willy Baccaglini, Marcello M. Gava, Prashant Patel, Sidney Glina
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Acknowledgment
After it gained notoriety, the CABEM project was supported by the university (FMABC/FUABC) and the local governments and health agencies of the involved municipalities-Santo André, São Bernardo do Campo, and São Caetano do Sul. The authors would like to especially acknowledge the efforts and support of Dr David Uip, Mrs Marina Mendonça, and the SUNO agency.
References
See the original publication
About the authors & affiliations
Fernando Korkes, MD, PhD1,2; Frederico Timóteo , MD1,2; Suelen Martins, MD3; Matheus Nascimento, MD1; Camila Monteiro , MD1; José H. Santiago, MD1; Willy Baccaglini, MD1,2; Marcel A. Silveira, MD1; Eduardo F. Pedroso, MD1; Marcello M. Gava, PhD1; Prashant Patel , MD4; Phillipe E. Spiess, MD5; and Sidney Glina, MD, PhD1
1Division of Urology, Faculdade de Medicina do ABC, Santo André, Brazil
2Hospital Municipal da Vila Santa Catarina and Hospital Israelita Albert Einstein, São Paulo, Brazil
3Division of Oncology, Faculdade de Medicina do ABC, Santo André, Brazil
4University of Birmingham, Birmingham, United Kingdom
5Moffit Cancer Center, Tampa, FL
Originally published at https://ascopubs.org.