Vaccines inhaled through the mouth or nose might stop the coronavirus in its tracks, although there’s little evidence from human trials so far.
Nature
Emily Waltz
06 September 2022
Site editor:
Joaquim Cardoso MSc.
Health Transformation Review
September 6, 2022
Are sprays the future of COVID-19 vaccines?
That’s the hope of dozens of research groups and companies working on new kinds of inoculation. Rather than relying on injections, these use sprays or drops administered through the nose or mouth that aim to improve protection against the virus SARS-CoV-2.
This week, an inhaled version of a COVID-19 vaccine, produced by the Chinese company CanSino Biologics in Tianjin, was approved for use as a booster dose in China.
It’s one of more than 100 oral or nasal vaccines in development around the world. In theory, these vaccines could prime immune cells in the thin mucous membranes that line cavities in the nose and mouth where SARS-CoV-2 enters the body, and quickly stop the virus in its tracks — before it spreads. Vaccine developers hope that these ‘mucosal’ vaccines will prevent even mild cases of illness and block transmission to other people, achieving what’s known as sterilizing immunity. A few mucosal vaccines are already approved for other diseases, including a sprayable vaccine against influenza.
Evidence in animals supports the idea that sterilizing immunity can be induced against COVID-19, although data from humans are scant.
Nature explains why mucosal vaccines might help to quash SARS-CoV-2, and what the latest findings mean.
Why might mucosal vaccines be better than conventional shots?
The COVID-19 vaccines currently in use do a good job of reducing disease severity and preventing hospitalization, but don’t block mild illness or transmission that well.
One reason is that they are injected into muscle. Intramuscular shots prompt an immune response that includes T cells, which destroy infected cells, and B cells, which produce antibodies that ‘neutralize’ pathogens — binding to them to stop them entering healthy cells. These cells and antibodies circulate through the bloodstream. But they aren’t present at high enough levels in the nose and lungs to provide rapid protection. In the time it takes for them to journey there from the bloodstream, the virus spreads, and the infected person gets ill.
Mucosal vaccines can prompt a whole-body immune response, but they can also activate immune cells in the mucosal tissue of the nose and respiratory tract. These localized cells “act as sentinels at the site of infection”, says Benjamin Goldman-Israelow, a physician-scientist at Yale School of Medicine in New Haven, Connecticut. “They can act much more quickly.”
The localized mucosal immune cells, known as tissue-resident memory T and B cells, have slightly different functions from the circulating T cells and B cells. For instance, tissue-resident memory B cells produce antibodies called secretory immunoglobulin A (IgA), which are intertwined with the layers of the respiratory tract, where they might be able to stop pathogens quickly. However, it is unclear how well secretory IgA will protect against SARS-CoV-2.
Researchers are testing mucosal vaccines as first doses for unvaccinated people and as boosters for those who have already received COVID-19 shots. Some mucosal vaccines are identical to injected vaccines, but are squirted as liquid or droplets up the nose. Others have a different composition, or are prepared differently. For instance, the mucosal vaccine developed by CanSino is the same as its injected one, but is packaged into aerosols and inhaled through the mouth with a nebulizer at one-fifth the dose of the injected version. A few mucosal vaccines in development are swallowed as pills.
How good are mucosal vaccines against other diseases?
At least nine mucosal vaccines are approved for use in people, against pathogens including poliovirus, influenza and cholera. Eight of these vaccines are taken orally, and one, against flu, is administered intranasally.
The oral polio vaccine, which induces immunity in the gut, is highly successful and comes close to achieving sterilizing immunity. In rare cases, however, this live attenuated vaccine will mutate and cause illness. For other diseases, mucosal vaccines haven’t been so successful — sometimes because the vaccine doesn’t generate a sufficiently strong immune response, and sometimes because it triggers side effects. The Swiss vaccine company Berna Biotech in Bern pulled its intranasal flu vaccine off the market in 2001, for instance, after discovering that it increased the risk of temporary facial paralysis.
A product called FluMist, a live attenuated intranasal vaccine against influenza that is approved in the United States and Europe, outperforms the intramuscular version in young children. Adults might also find it more convenient to have a vaccine sprayed up the nose, rather than injected. But FluMist hasn’t worked as well in adults. That’s because many have had years to build up some immunity to flu viruses. Even if this immunity isn’t strong enough to prevent the disease, adults’ mucosal immune responses might still block the attenuated vaccine from infecting nasal cells, or clear it before it has a chance to do its job.
“It’s a balancing act between making sure the vaccine doesn’t cause illness, and yet replicates enough to elicit mucosal immunity in people who have had some experience with the virus,” says Kanta Subbarao, director of the World Health Organization (WHO) Collaborating Centre for Reference and Research on Influenza in Melbourne, Australia. Researchers don’t yet know if this issue might also affect COVID-19 intranasal vaccines.
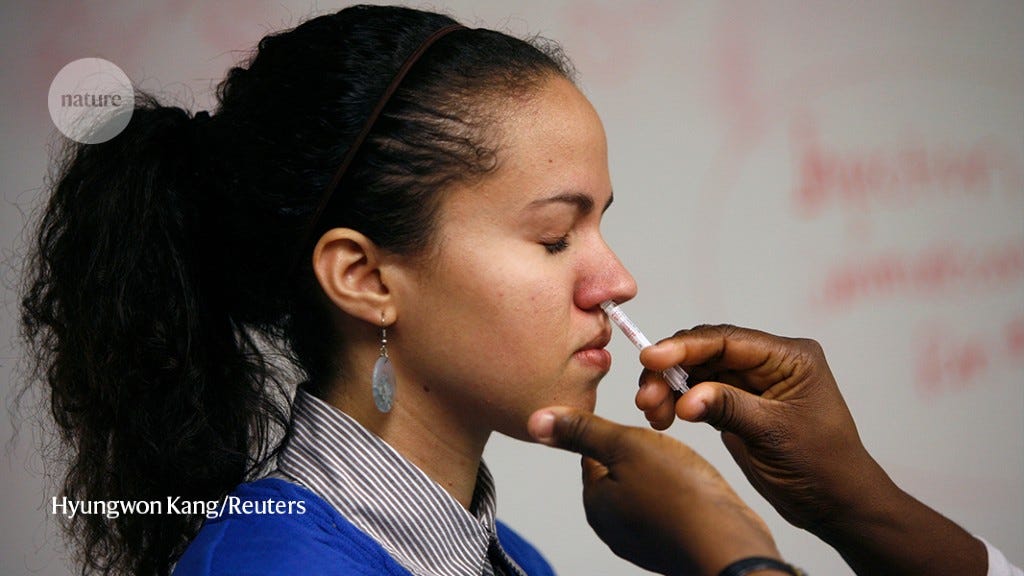
Where and when might mucosal COVID-19 vaccines be available?
Around 100 mucosal COVID-19 vaccines are in development globally, according to Airfinity, a health-analytics company in London (see ‘Mucosal COVID-19 vaccines’). Around 20 of those have reached clinical trials in humans, of which at least four — in India, Iran and two in China — have completed or are undergoing phase III studies to test safety and how well they work compared to other vaccines. Iran gave its vaccine emergency approval in October 2021, and at least five million doses have been delivered to the Ministry of Health, says Ali Es-haghi, an analytical chemist at the Razi Vaccine and Serum Research Institute in Karaj, which developed the vaccine. But the institute has not yet published data on efficacy in humans. (Russia is said to have approved a mucosal vaccine for its market but has not published data, and the vaccine makers did not respond to Nature ‘s request for details.)
Large-scale human trial data on mucosal vaccines in the United States and Europe will take another year or two. “There’s not the same sense of urgency now” compared with at the beginning of the pandemic, says Louise Blair, head of vaccines and variants at Airfinity. “We are in an abundance of vaccines. Countries at the moment seem to be satisfied with protection against hospitalization rather than infection. So funding and resources are very different, and I don’t think we’ll see the same speed of development,” she says.
In the meantime, countries must rely on intramuscular boosters to maintain immunity. Some public-health authorities are updating boosters against coronavirus variants such as Omicron, although early data suggest that these perform only slightly better than an extra dose of older vaccines. But relying solely on boosters to suppress variants “may not be the optimal approach”, says Robert Seder, chief of cellular immunology at the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland. “To increase protection against transmission, we may need to change the delivery” of boosters to increase mucosal responses, he says.
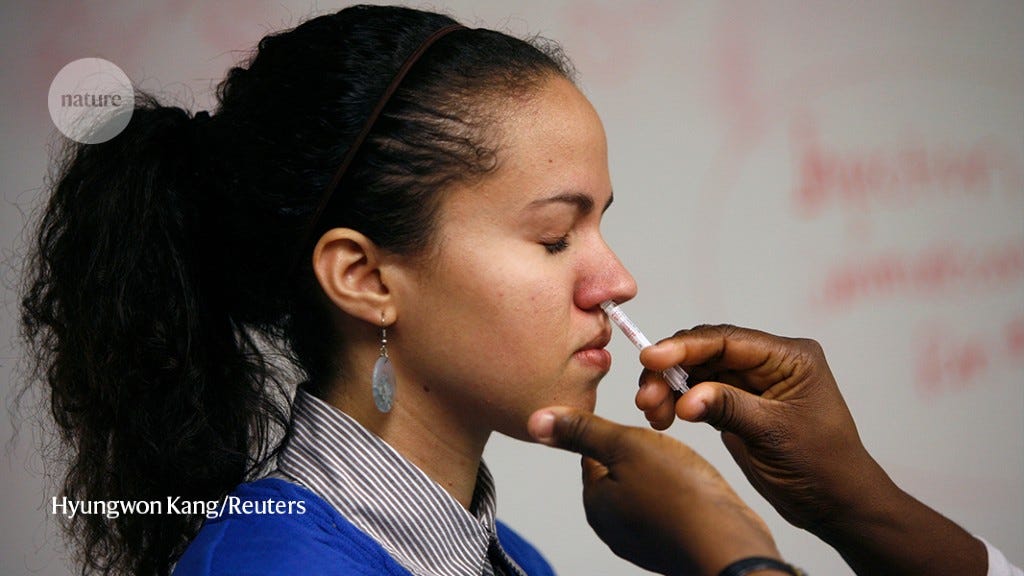
Can mucosal COVID-19 vaccines induce sterilizing immunity?
Preventing infection and transmission is a high bar for any vaccine. But studies of SARS-CoV-2 mucosal vaccines in animals suggest that it’s possible. For example, a study in mice by Goldman-Israelow and his colleagues at Yale University found that an intranasal booster (given after one dose of conventional vaccine) induced mucosal immunity and completely protected the animals from a lethal level of exposure to the coronavirus, whereas an intramuscular booster did not.
And in rhesus macaques ( Macaca mulatta), another intranasal vaccine — which used an influenza-like virus to deliver SARS-CoV-2 RNA to cells — completely protected the animals from SARS-CoV-2 infection. Virus replication was undetectable in the monkeys’ airways and lung tissues, says Ursula Buchholz, chief of the RNA viruses section at the NIAID, who led the study. “In preclinical models, we get something that’s very close to sterilizing immunity. We’ll have to see how this translates into clinical studies,” she says.
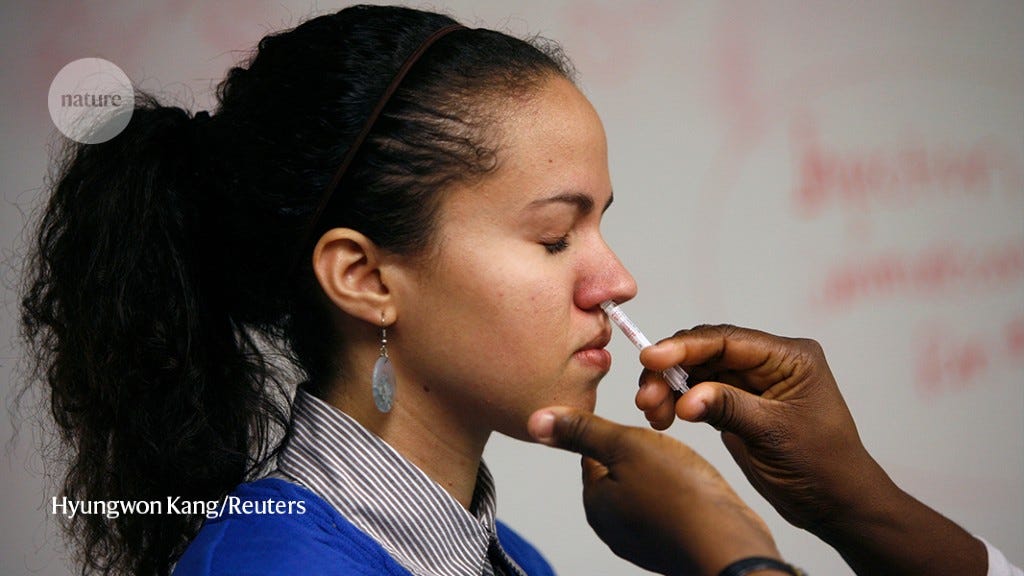
How are researchers measuring the efficacy of mucosal vaccines in people?
There’s a quick way to predict whether an intramuscular COVID-19 vaccine will be effective: measure the neutralizing-antibody levels circulating in the blood. Higher levels generally mean better protection — something researchers have established after decades of experience with intramuscular vaccines against other pathogens.
But for mucosal vaccines that aim to induce sterilizing immunity, no clear-cut correlate exists. Many developers are measuring immune responses in the respiratory tract, including secretory IgA, other antibodies and tissue-resident memory T cells. These probably contribute to protection, but it’s unclear what levels are necessary to prevent infection and transmission. Studies of immune responses in the nose and lungs of people who have experienced a natural infection might prove informative.
Until this basic research is established, mucosal-vaccine developers must determine efficacy in other ways. The company Bharat Biotech in Hyderabad, India, for instance, measured systemic neutralizing antibodies in blood serum in its trial of an intranasal COVID-19 vaccine. If those match or exceed the antibody levels of intramuscular vaccines on the market, the trial will achieve its primary endpoint and be considered a success. But it will not determine the vaccine’s ability to prevent infection or transmission. Last month, the firm said it had sent late-stage testing data — as yet unpublished — to the country’s regulator, hoping for approval to start providing the vaccine to clinics.
CanSino tracked efficacy using a similar strategy — measuring the levels of neutralizing antibodies in blood serum and comparing them to those from existing vaccines. A phase II study of the company’s aerosolized mucosal vaccine reported in January that, when given as a booster, the vaccine raised serum antibody levels significantly more than did a boost from CanSino’s intramuscular vaccine. In July, the firm noted in a further report that antibody levels waned over time, but were still higher than those elicited through the intramuscular route. The company is also measuring T cells and antibodies in saliva, but the levels of response needed to provide sterilizing immunity aren’t known.
The Chinese firm Beijing Wantai Biological Pharmacy also has a mucosal vaccine in phase III trials, but the company did not respond to Nature ‘s request for comment.
Another option is to conduct efficacy studies by comparing a mucosal vaccine against a placebo group. Codagenix in Farmingdale, New York, and the Serum Institute of India in Pune are taking this approach in a phase II/III study of an intranasal vaccine in 20,000 unvaccinated people, about half of whom will receive a placebo in their noses. Efficacy will be determined by comparing the number of confirmed cases in each group and measuring the rate of protection from the vaccine, says Robert Coleman, chief executive at Codagenix.
Placebo groups are getting harder to assemble as the number of people who haven’t been infected with SARS-CoV-2 or vaccinated dwindles. Such trials are also hard to justify ethically when effective vaccines are readily available. However, there are countries that have low vaccination rates and limited vaccine access, where such a trial can be conducted ethically. Codagenix’s phase II/III study is part of the WHO’s Solidarity Trial Vaccines, which brings several trials together to share one placebo group. A Codagenix spokesperson says that trials are being conducted in countries in Africa to start with, but did not disclose details. They aren’t expected to yield results until 2023. (Codagenix is also working on a trial of its intranasal vaccine as a booster, currently in phase I.)
“It’s completely possible to determine efficacy,” says Sandy Douglas, who is chief investigator of an intranasal SARS-CoV-2 vaccine being developed by the University of Oxford, UK. “It’s just a bit trickier than testing first-generation intramuscular vaccines in an infection-naive population,” he says.
doi: https://doi.org/10.1038/d41586-022-02824-3
Originally published at https://www.nature.com on September 6, 2022.