Lack of Equitable Representation in Clinical Trials Compounds Disparities in Health and Will Cost U.S. Hundreds of Billions of Dollars; Urgent Actions Needed by NIH, FDA, Others to Boost Representation
National Academies
News Release
May 17, 2022
A new congressionally mandated report from the National Academies of Sciences, Engineering, and Medicine calls for urgent actions by federal agencies, Congress, journals, and others to improve the representation of racial and ethnic minority groups and other underrepresented populations in clinical trials and research.
Currently, lack of representation in research is compounding disparities in health outcomes, with serious consequences for underrepresented groups and the nation as a whole, said the committee that wrote the report.
Currently, lack of representation in research is compounding disparities in health outcomes, with serious consequences for underrepresented groups and the nation as a whole, said the committee that wrote the report.
“While U.S. investments in clinical research have contributed significantly to treating and preventing disease and extending human life, large swaths of the population are less able to benefit from these discoveries because they are not adequately represented in clinical research studies,” said committee chair Kirsten Bibbins-Domingo, chair of the department of epidemiology and biostatistics and professor of medicine at the University of California, San Francisco.
“As the U.S. becomes more diverse every day, failing to reach these growing communities will only prove more harmful and costly over time.”
“While U.S. investments in clinical research have contributed significantly to treating and preventing disease and extending human life, large swaths of the population are less able to benefit from these discoveries because they are not adequately represented in clinical research studies …
“As the U.S. becomes more diverse every day, failing to reach these growing communities will only prove more harmful and costly over time.”
Improving representation in research would help to reduce disparities in health outcomes and the harm they cause, along with their economic toll, said the committee.
Improving representation in research would help to reduce disparities in health outcomes and the harm they cause, along with their economic toll, said the committee.
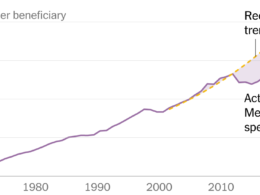
The High Health and Economic Costs of Underrepresentation
An equitable clinical research enterprise would include trials and studies that match the demographics of the disease burden under study, but the nation remains far from achieving this goal, the report says.
Although there has been progress on some fronts — particularly with representation of White women in clinical trials and research — it has largely stalled on participation of racial and ethnic minority population groups.
In addition, older adults, pregnant and lactating individuals, LGBTQIA+ populations, and people with disabilities remain underrepresented and even excluded from clinical trials and clinical research.
An equitable clinical research enterprise would include trials and studies that match the demographics of the disease burden under study, but the nation remains far from achieving this goal …
Although there has been progress on some fronts — particularly with representation of White women in clinical trials and research — it has largely stalled on participation of racial and ethnic minority population groups.
This underrepresentation has serious consequences, the report says, including:
- Lack of representation may lead to lack of access to effective medical interventions. Approval and indications for new therapeutics are often restricted to the demographics of the populations included in the clinical studies. Lack of representation may therefore impede access to a specific therapeutic agent for some groups of patients.
- Lack of representation compounds health disparities in the populations currently underrepresented and excluded in clinical trials and clinical research. While achieving health equity and reducing health disparities requires far more than just equitable representation in clinical research, failure to achieve this leaves these disparities unaddressed and reinforces inequities.
- Lack of representation costs hundreds of billions of dollars.
An economic analysis carried out by the committee demonstrates high financial and social costs — measured by life expectancy, disability-free life, and years in the labor force — projected to be in the hundreds of billions of dollars range over the next three decades.
If better representation in clinical trials reduces health disparities by even a modest amount, the analysis found that achieving diverse representation in research would be worth billions of dollars in savings to the United States.

Underrepresented Populations Willing to Participate, If Asked
Distrust and mistrust are commonly assumed to be the reason underlying lack of participation in clinical trials, the report notes.
While there is no doubt that the legacy of abuses in medical research is an important factor driving the lack of engagement of underrepresented populations with research, several studies have found that distrust and mistrust are not necessarily associated with a lack of willingness to participate.
Evidence shows that Asian, Black, and Latinx Americans, and American Indian/Alaska Native individuals are no less likely, and in some cases are more likely, to participate in research if asked, the report says.
Evidence shows that Asian, Black, and Latinx Americans, and American Indian/Alaska Native individuals are no less likely, and in some cases are more likely, to participate in research if asked…

Actions Needed to Drive Systemic Change
Improving representation is the responsibility of everyone involved in the clinical research enterprise, and a range of steps are needed to change the nation’s approach to clinical research to more equitably recruit and retain a diverse group of participants, the report says.
Improving representation is the responsibility of everyone involved in the clinical research enterprise, …
… and a range of steps are needed to change the nation’s approach to clinical research to more equitably recruit and retain a diverse group of participants…
Its recommendations focus on system-level actions to drive change on a broad scale.
Among the report’s recommendations:
- The Food and Drug Administration should require study sponsors to submit a detailed recruitment plan no later than at the time of submission of the Investigational New Drug and Investigational Device Exemption application that explains how they will ensure that the trial population appropriately reflects the demographics of the disease or condition under study.
- The Office of Human Research Protections (OHRP) and the FDA should direct local institutional review boards (IRBs) to assess and report the representativeness of clinical trials as one measure of sound research design that it requires for the protection of human subjects.
- To determine how to take action on the most effective accountability and incentive structures, Congress should direct FDA to enforce existing accountability measures and establish a taskforce to study new incentives for new drug and device trials that achieve representative enrollment.
Some ideas include tax incentives, fast-track criteria and exemption from some FDA drug application fees, extended market exclusivity to sponsors who meet predefined criteria of representativeness, and refusing to file an application that does not appropriately represent the target population under study. - In grant proposal review, the NIH should formally incorporate considerations of participant representativeness in the score-driving criteria that assess the scientific integrity and overall impact of a grant proposal.
- Journal editors, publishers, and the International Committee on Medical Journal Editors should require information on the representativeness of trials and studies for submissions to their journals, particularly relative to the affected population; should consider this information in accepting submissions; and should publish this information for accepted manuscripts.
“While we all bear the cost of excluding women, racial and ethnic minority groups, LGBTQIA+ populations, people with disabilities, older adults, and pregnant and lactating individuals from clinical trials, the populations left out of research bear the greatest cost, as they may lose out on benefiting from the United States’ substantial investment in scientific advancement and may be deprived of access to novel treatments,” said Victor J. Dzau, president of the National Academy of Medicine.
“All stakeholders in the research enterprise must take committed and accountable actions in order for our country to have a diverse, inclusive clinical research portfolio.”
“All stakeholders in the research enterprise must take committed and accountable actions in order for our country to have a diverse, inclusive clinical research portfolio.” — Victor J. Dzau, president of the National Academy of Medicine.
The study — undertaken by the Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research — was sponsored by the National Institutes of Health.
The National Academies of Sciences, Engineering, and Medicine are private, nonprofit institutions that provide independent, objective analysis and advice to the nation to solve complex problems and inform public policy decisions related to science, technology, and medicine.
They operate under an 1863 congressional charter to the National Academy of Sciences, signed by President Lincoln.
Some statistics about the report (statnews)
The report cited many studies that show a lack of diversity persists in clinical trials — even for diseases that disproportionately impact non-white populations. One Food and Drug Administration analysis of clinical trials conducted between 2015 and 2018 showed that 78% of participants were non-Hispanic white people. More than 97% of participants in a Phase 2 trial of the Alzheimer’s drug crenezumab were white and just 2.8% were Hispanic even though Hispanic people are 1.2 times more likely to develop Alzheimer’s, the report said.
This lack of diversity has stubbornly persisted despite decades of attention to the issue, dozens of reports, and the creation of new offices in federal agencies to encourage broader participation in trials. A 2015 Government Accountability Office report found little improvement in trial diversity had occurred since 2004. To this day, the new report said, “research participants remain mostly white and male.”
The report’s authors noted that diversity targets for clinical trials are seldom enforced, and they urged better tracking of the diversity of trial participants, withholding of funding for trials that do not meet goals, and offering tax credits or other financial incentives for those who do increase representation.
To illustrate why diversity in clinical trials is critical, the report’s authors cited the blood clot inhibitor warfarin as a cautionary tale. While the drug has been approved for use since 1951, it was not until 2013 that scientists realized genetic ancestry impacted how the drug should be dosed. Many people with Asian ancestry require lower doses and suffered excess bleeding when the drug was used at dosages set after trials conducted on mostly white men, the report said.
The authors cited other serious issues caused by the lack of diversity, including the inability to generalize research to the larger U.S. population; difficulty recruiting enough participants for many studies, which is the leading cause of clinical trial failure; and a lack of trust in the clinical research and medical establishments by those who have been historically excluded from clinical trials.
The Business Case (statnews)
An economic analysis commissioned as part of the report showed that billions could be saved by reducing the harms caused by diabetes and heart disease if just 1% of health disparities were reduced though more diverse clinical trials. “As the U.S. becomes more diverse every day, failing to reach these growing communities will only prove more harmful and costly over time,” the report’s chair, Kirsten Bibbins-Domingo, said in a statement.
The cost of these disparities goes beyond dollars.”While it’s easy to calculate the economic costs, it’s impossible to fully calculate the emotional trauma associated with inequities in health care,” committee member Jason Resendez, president and CEO of the National Alliance for Caregiving, told STAT.

Featured Report
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups
The United States has long made substantial investments in clinical research with the goal of improving the health and well-being of our nation. There is no doubt that these investments have contributed significantly to treating and preventing disease and extending human life. Nevertheless, clinical research faces a critical shortcoming. Currently, large swaths of the U.S. population, and those that often face the greatest health challenges, are less able to benefit from these discoveries because they are not adequately represented in clinical research studies. While progress has been made with representation of white women in clinical trials and clinical research, there has been little progress in the last three decades to increase participation of racial and ethnic minority population groups. This underrepresentation is compounding health disparities, with serious consequences for underrepresented groups and for the nation.
At the request of Congress, Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups identifies policies, procedures, programs, or projects aimed at increasing the inclusion of these groups in clinical research and the specific strategies used by those conducting clinical trials and clinical and translational research to improve diversity and inclusion. This report models the potential economic benefits of full inclusion of men, women, and racial and ethnic groups in clinical research and highlights new programs and interventions in medical centers and other clinical settings designed to increase participation.
SUGGESTED CITATION
Improving Representation in Clinical
Trials and Research: Building Research
Equity for Women and Underrepresented
Groups (2022)
440 pages | 6 x 9 | PAPERBACK
ISBN 978-0-309-27820-1 | DOI 10.17226/26479
CONTRIBUTORS
Kirsten Bibbins-Domingo and Alex Helman,
Bibbins-Domingo, a professor at the University of California, San Francisco, and the incoming editor of the Journal of the American Medical Association, said the issue of representation in clinical trials became urgent for her in 2017, when she chaired the U.S. Preventive Services Task
Force and found few studies to help her assess how guidelines should differ for racial and ethnic populations with higher medical risk factors or medical needs.
It was also the year her father died of prostate cancer. While the cancer has an incidence 75% higher in Black men, Bibbins-Domingo found that Black men made up fewer than 5% of enrollees in prostate cancer clinical trials and fewer than 2.5% of those in late-stage clinical trials, a disparity she called “acutely distressing.”
Editors; Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research; Committee on Women in Science, Engineering, and Medicine; Policy and
Global Affairs; National Academies of Sciences, Engineering, and Medicine
National Academies of Sciences, Engineering, and Medicine 2022. Improving
Representation in Clinical Trials and Research: Building Research Equity for
Women and Underrepresented Groups. Washington, DC: The National Academies
Press. https://doi.org/10.17226/26479.
COMMITTEE ON IMPROVING THE REPRESENTATION OF
WOMEN AND UNDERREPRESENTED MINORITIES IN
CLINICAL TRIALS AND RESEARCH
Kirsten Bibbins-Domingo, M.D., Ph.D., M.A.S. (Chair) (NAM),1 Professor and Chair of the
Department of Epidemiology and Biostatistics and Lee Goldman, MD Endowed Chair and
Professor of Medicine, University of California, San Francisco; Inaugural Vice Dean for
Population Health and Health Equity, UCSF School of Medicine
Marcella Alsan, M.D., Ph.D., M.P.H., Professor of Public Policy, Harvard Kennedy School;
Co-Director of the Health Care Delivery Initiative Abdul Latif Jameel Poverty Action Lab,
Massachusetts Institute of Technology
Arleen Brown, M.D., Ph.D., Professor of Medicine, University of California, Los Angeles;
Chief of General Internal Medicine and Health Services Research, Olive View-UCLA Medical
Center
Gloria Coronado, Ph.D., Epidemiologist and Mitch Greenlick Endowed Distinguished
Investigator in Health Disparities Research, Kaiser Permanente Center for Health Research
Carlos del Rio, M.D. (NAM), Distinguished Professor of Medicine, Emory University School
of Medicine; Professor of Epidemiology and Global Health, Rollins School of Public Health,
Emory University; Executive Associate Dean for Emory University School of Medicine,
Grady Health System
XinQi Dong, M.D., M.P.H., Director of the Institute for Health, Health Care Policy, and Aging
Research (IFH) and Inaugural Henry Rutgers Professor of Population Health Sciences,
Rutgers University
Dana Goldman, Ph.D. (NAM), Dean of the Sol Price School of Public Policy, C. Erwin and
Ione L. Piper Chair, and Leonard D. Schaeffer Director’s Chair, Schaeffer Center for Health
Policy & Economics, University of Southern California
1 Designates membership in the National Academy of Sciences (NAS), National Academy of Engineering
(NAE), or National Academy of Medicine (NAM).
PREPUBLICATION COPY—Uncorrected Proofs
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups
Copyright National Academy of Sciences. All rights reserved.
vi
Sharon K. Inouye, M.D., M.P.H. (NAM), Professor of Medicine, Harvard Medical School;
Milton and Shirley F. Levy Family Chair, and Director, Aging Brain Center, Marcus Institute
for Aging Research, Hebrew SeniorLife
Jonathan Jackson, Ph.D.,2 Executive Director of Community Access, Recruitment, and
Engagement (CARE) Research Center, Massachusetts General Hospital and Harvard
Medical School
Amelia Knopf, Ph.D., M.P.H., Assistant Professor of Nursing, Indiana University
Edith A. Perez, M.D., Chief Medical Officer of Bolt Therapeutics, Inc.; Professor of Medicine,
Mayo Clinic; Director of the Mayo Clinic Breast Cancer Translational Genomics Program
Phyllis Pettit Nassi, B.S., M.S.W., Associate Director for Research and Science, Special
Populations, American Indian Program, Huntsman Cancer Institute, University of Utah
Jason Resendez, B.A., President and CEO, National Alliance for Caregiving
Susan Schaeffer, B.F.A., Founder, President, and CEO of The Patients’ Academy for
Research Advocacy
Study Staff
Alex Helman, Ph.D., Study Director and Program Officer, Committee on Women in Science,
Engineering, and Medicine
Ashley Bear, Ph.D., Acting Director, Committee on Women in Science, Engineering, and
Medicine
Laura Aiuppa, M.S., Senior Program Officer, Board on Health Care Services
Austen Applegate, Research Associate, Committee on Women in Science, Engineering, and
Medicine
John Veras, Senior Program Assistant, Committee on Women in Science, Engineering, and
Medicine (August 2020 to February 2022)
Abigail Harless, Senior Program Assistant, Committee on Women in Science, Engineering,
and Medicine (from February 2022)
2 Dr. Jonathan Jackson resigned from the Committee on Improving Representation of Women and
Underrepresented Minorities in Clinical Trials and Research, effective June 2, 2021.
PREPUBLICATION COPY—Uncorrected Proofs
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups
Copyright National Academy of Sciences. All rights reserved.
vii
Mollie Marr, Mirzayan Fellow, Committee on Women in Science, Engineering, and
Medicine
Anne Marie Houppert, MSLS, Senior Librarian
Consultants
Franchesca Arias, Ph.D., Instructor in Neurology and Assistant Scientist, Harvard Medical
School
Adam Bress, PharmD., M.S., National Academy of Medicine Fellow in Pharmacy
Brandon Brown, Ph.D., M.P.H., Associate Professor, University of California, Riverside
Farah Acher Kaiksow, M.D., M.P.P., Assistant Professor of Medicine, Harvard Medical
School
Aishwarya Bhattacharya, M.P.H., Student Research Assistant, Yale School of Public Health
Laura Bothwell, Ph.D., M.Phil., M.A., Associate Research Scientist, Yale School of Public
Health
Jocelyn Carter, M.D., M.P.H., Professor of Medicine, Harvard Medical School
Amber Datta, M.P.H., Student Research Assistant, Yale School of Public Health
Jakub Hlávka, Ph.D., Fellow, USC Schaeffer Center for Health Policy & Economics;
Research Assistant Professor, Health Policy and Management, USC Price School of Public
Policy; Research Fellow, USC Center for Risk and Economic Analysis of Terrorism Events
(CREATE)
Aaron Kesselheim, M.D., J.D., M.P.H., Professor of Medicine, Harvard Medical School
Amy J. H. Kind, M.D., Ph.D., Professor of Medicine, University of Wisconsin School of
Medicine and Public Health
Niroop Rajashekar, B.S., Student Research Assistant, Yale School of Medicine
Nicole Rogus-Pulia, Ph.D., M.A., Assistant Professor of Medicine and Surgery, University
of Wisconsin School of Medicine and Public Health
Bryan Tysinger, Ph.D., Director of Health Policy Microsimulation, USC Schaeffer Center for
Health Policy & Economics; Fellow, USC Schaeffer Center; Research Assistant Professor,
USC Price School of Public Policy
PREPUBLICATION COPY—Uncorrected Proofs
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups
Copyright National Academy of Sciences. All rights reserved.
viii
Leslie Wang, M.S., Student Research Assistant, Yale School of Medicine
RELATED ARTICLES