The Health Transformation
institute for health strategy, digital health
and continuous health transformation
Joaquim Cardoso MSc
Chief Research and Strategy Officer (CRSO)
May 16, 2023
Adapted from on “Quick Blood Tests to Spot Cancer: Will They Help or Harm Patients?”, published on the Financial Times, on the 17th of May, 2023.
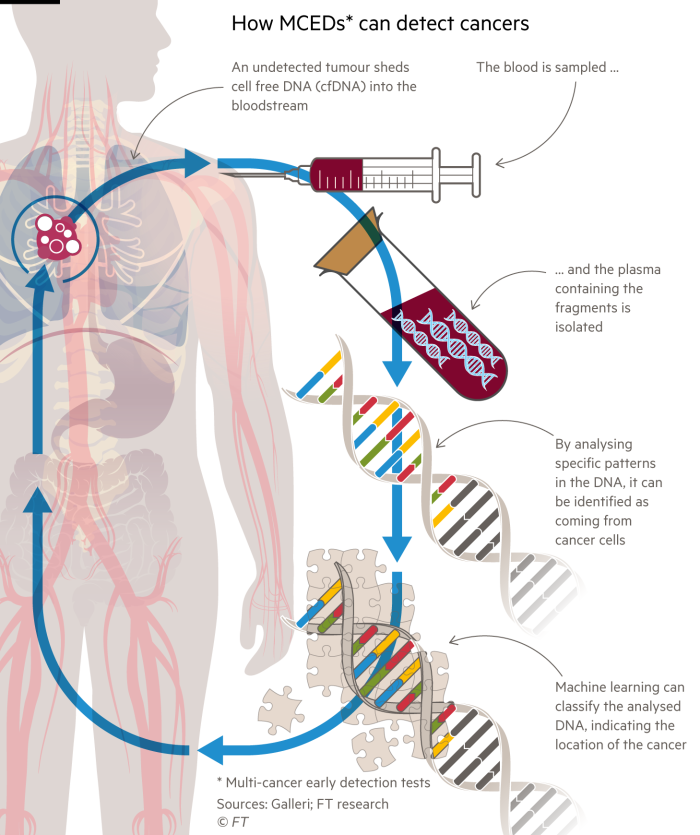
Multi-cancer early detection tests (MCED) are being developed to analyze DNA fragments in the blood to detect multiple types of cancer from a single blood draw.
Health bodies in the US and UK are conducting clinical trials for MCED tests, with hopes of integrating them into national screening programs. Companies like Grail and Datar Cancer Genetics are already selling MCED tests to the public, but they have not yet received approval from regulatory bodies like the US Food and Drug Administration.
The MCED market is projected to reach $23 billion by 2031, attracting significant investment.
Critics raise concerns about the potential risks associated with misdiagnosis, over-diagnosis, and over-treatment, which may harm patients. The recent Theranos scandal has made investors cautious about bold claims surrounding blood tests.
Delays in regulatory approval and the need for extensive trials before marketing the tests are also a point of contention. Screening for certain types of cancer can lead to false-positive results, unnecessary tests, and treatment with potential risks and anxiety. Slow-growing tumors and over-diagnosis are additional concerns associated with cancer screening.
The Galleri test, one of the MCED tests, has shown mixed results in detecting cancer, missing some cases and producing false positives.
Some doctors and experts believe that the rollout of MCED tests should be accompanied by long-term studies to prove their effectiveness in saving lives. Firefighters in Arizona who underwent MCED tests experienced missed cancer cases, prompting doubts about the test’s reliability. Despite the potential benefits of MCED tests, there are concerns that their widespread use may not significantly impact cancer mortality rates and may strain healthcare systems.
Further research and testing are required before MCED tests can be considered a reliable and safe screening method.
Statistics:
- The MCED market is expected to expand from $1.5 billion in revenues in 2021 to $23 billion by 2031.
- Grail’s study of almost 6,700 participants found that false positives accounted for almost a third of the cancer signals detected, leading to invasive procedures.
- About 20–30% of breast cancers are missed by mammogram screenings.
- One in eight mammograms produces a false-negative result.
Examples:
- Valerie Caro’s life was saved by the Galleri blood test, which detected gallbladder cancer early.
- The UK’s National Health Service (NHS) is participating in a clinical trial of the Galleri test involving 140,000 patients.
- Grail and Datar Cancer Genetics are already selling MCED tests to the public.