This is an excerpt of the paper “Priorities for cancer research in low- and middle-income countries: a global perspective”, published on Nature Medicine, by C. S. Pramesh, Rajendra A. Badwe, Elisabete Weiderpass et al, on 19 April 2022, focusing on the topic above.
The excerpt is preceded by an Executive Summary, by the author of the blog. For the full version of the original publication, please refer to the 2nd part of this post.
Nature Medicine
C. S. Pramesh, Rajendra A. Badwe, Elisabete Weiderpass et al,
19 April 2022
Executive Summary
by Joaquim Cardoso MSc.
Health Revolution Institute
Cancer Revolution Unit
April 27, 2022
Cancer research is heavily skewed toward HICs, with disproportionately less research conducted in, and relevant to, the problems of LMICs.
The study identified five top priorities in cancer research in LMICs based on current and projected needs:
- reducing the burden of patients with advanced disease;
- improving access and affordability, and outcomes of cancer treatment;
- value-based care and health economics;
- quality improvement and implementation research; and
- leveraging technology to improve cancer control.
LMICs have an excellent opportunity to address important questions in cancer research that could impact cancer control globally.
EXCERPT
5.Leveraging technology to improve cancer control
Technology has advanced considerably in the fields of molecular biology, next-generation sequencing, precision medicine, robotics, advanced imaging and radiation, to name but a few.
However, for the most part, these advances are not directly applicable to patients in LMICs over the near term.
Technology has advanced considerably in the fields of molecular biology, next-generation sequencing, precision medicine, robotics, advanced imaging and radiation, to name but a few.
However, for the most part, these advances are not directly applicable to patients in LMICs over the near term.
Less studied is the intersection between technology and medicine in addressing pressing health care (including cancer) problems in LMICs.
Interdisciplinary research involving physicians, researchers, medicinal chemists, scientists, engineers, experts in digital health, artificial intelligence and machine learning has the potential to identify problems and find solutions that are well suited to LMIC contexts.
Such collaboration could address the four thematic areas of research described earlier.
Less studied is the intersection between technology and medicine in addressing pressing health care (including cancer) problems in LMICs.
Examples of technology-enabled research that could address real-world problems in LMICs include
- point-of-care diagnostics (lab-on-a-chip),
- telemedicine solutions (including telepathology and teleradiology),
- image analysis and pattern recognition for pathology and radiology,
- virtual reality in training,
- patient care pathways and
- digital applications for patient-reported outcomes, among others.
Examples of technology-enabled research that could address real-world problems in LMICs … are abundant …
However, these new, intuitively appealing technologies must be validated in real-life situations.
However, these new, intuitively appealing technologies must be validated in real-life situations.
Previous instances of clinical decision-support systems that promised more than they delivered illustrate how technology can overtake the scientific method.
Similarly, several digital applications have been launched without appropriate research to validate their performance.
The Affordable Cancer Technologies program of the NCI is a good example of focusing efforts on technology-enabled solutions along with validation in LMICs ( https://www.cancer.gov/about-nci/organization/cgh/research-training-programs/affordable-cancer-technology).
With the caveat of requiring rigorous evaluation, judicious use of technology has the potential to enable LMICs to achieve much-improved health outcomes.
ORIGINAL PUBLICATION (executive summary)

Priorities for cancer research in low- and middle-income countries: a global perspective
Nature Medicine
C. S. Pramesh, Rajendra A. Badwe, Elisabete Weiderpass et al,
19 April 2022
mskcc
Executive Summary
by Joaquim Cardoso MSc.
Health Revolution Institute
Cancer Revolution Unit
April 27, 2022
Cancer research is heavily skewed toward HICs, with disproportionately less research conducted in, and relevant to, the problems of LMICs.
- Survival rates of cancer are strikingly lower in LMICs than in HICs.
- While this may be due partly to more non-lethal cancers being identified by population-level screening in HICs, the more substantive reason is the variation in the quality of care provided in LMICs.
- There is failure to implement many interventions that are proven to be cost effective, even in HICs.
- In the US and Europe, 30–50% of patients do not receive optimal evidence-based clinical interventions, and this number is likely much higher in LMICs.
There is a strong economic argument supporting cancer research:
- every million dollars spent on cancer research in the US are estimated to produce value worth 28 million dollars in the following 50 years
- In the UK, every pound invested in cancer research generated 0.4 £ per year thereafter.
While ongoing efforts focus predominantly on expansion and strengthening of treatment facilities, relatively less attention is paid to generating country-specific evidence for effective prevention, early detection, access, survivorship and palliation, with an emphasis on quality and value.
Several gaps in the research enterprise of LMICs need to be addressed to promote regionally relevant research. These include :
- the scarcity of reliable data
- a paucity of clinical trials and
- a lack of an environment conducive to research in academic institutions, including resea
The study identified five top priorities in cancer research in LMICs based on current and projected needs:
- reducing the burden of patients with advanced disease;
- improving access and affordability, and outcomes of cancer treatment;
- value-based care and health economics;
- quality improvement and implementation research; and
- leveraging technology to improve cancer control.
LMICs have an excellent opportunity to address important questions in cancer research that could impact cancer control globally.
Governments, policy makers, funding agencies, health care organizations and leaders, researchers and the public should work together and show strong commitment to promote cancer research in LMICs.
ORIGINAL PUBLICATION (full version)

Abstract
Nature Medicine
C. S. Pramesh, Rajendra A. Badwe, Elisabete Weiderpass et al,
19 April 2022
Cancer research currently is heavily skewed toward high-income countries (HICs), with little research conducted in, and relevant to, the problems of low- and middle-income countries (LMICs).
This regional discordance in cancer knowledge generation and application needs to be rebalanced.
Several gaps in the research enterprise of LMICs need to be addressed to promote regionally relevant research, and radical rethinking is needed to address the burning issues in cancer care in these regions.
We identified five top priorities in cancer research in LMICs based on current and projected needs:
- reducing the burden of patients with advanced disease;
- improving access and affordability, and outcomes of cancer treatment;
- value-based care and health economics;
- quality improvement and implementation research; and
- leveraging technology to improve cancer control.
LMICs have an excellent opportunity to address important questions in cancer research that could impact cancer control globally.
Success will require collaboration and commitment from governments, policy makers, funding agencies, health care organizations and leaders, researchers and the public.

Main
LMICs face a double burden of disease, with non-communicable diseases, including cancer, rising rapidly alongside continued morbidity and mortality from infectious diseases.
While age-standardized rates of cancer have changed only marginally, the absolute number of patients diagnosed with cancer annually in LMICs is growing rapidly.
By 2030, approximately three-quarters of all cancer deaths will occur in LMICs, with one in eight people experiencing a cancer diagnosis in their lifetime.
By 2030, approximately three-quarters of all cancer deaths will occur in LMICs, with one in eight people experiencing a cancer diagnosis in their lifetime.
Most of the increase in the global cancer burden in the next 50 years will come from LMICs (400% in low-income countries, 168% in middle-income countries and 53% in HICs) due to rising population, increasing life expectancy, growing urbanization and lifestyle changes.
Although age-standardized incidence rates for cancer are lower in LMICs than in HICs, the mortality:incidence ratio is higher in LMICs.
Efforts for cancer control in LMICs should aim to reduce exposure to common modifiable risk factors such as tobacco, alcohol and obesity, improve access to care and improve outcomes for those diagnosed.
While ongoing efforts focus predominantly on expansion and strengthening of treatment facilities, relatively less attention is paid to generating country-specific evidence for effective prevention, early detection, access, survivorship and palliation, with an emphasis on quality and value.
Efforts for cancer control in LMICs should aim to reduce exposure to common modifiable risk factors such as tobacco, alcohol and obesity, improve access to care and improve outcomes for those diagnosed.
While ongoing efforts focus predominantly on expansion and strengthening of treatment facilities, relatively less attention is paid to generating country-specific evidence for effective prevention, early detection, access, survivorship and palliation, with an emphasis on quality and value.
Cancer research is heavily skewed toward HICs, with disproportionately less research conducted in, and relevant to, the problems of LMICs.
For example, of all phase 3 trials of anti-cancer therapies conducted worldwide between 2014 and 2017, only 8% were initiated and conducted in LMICs, despite increasing recognition that trial results are not necessarily generalizable across populations and country contexts.
The gross imbalance in cancer knowledge generation and application through the global research enterprise raises several issues.
First, research and innovation conducted in HICs fail to adequately address certain cancers that are prevalent in LMICs, for example, oral, esophagogastric, hepatobiliary and cervical cancers.
Second, cancer-control strategies that are effective in HICs are often not applicable to LMICs as a result of differences in disease characteristics, health systems capacities, sociocultural factors, treatment-completion rates, lack of availability of medicines, and pharmacokinetic and biological variation associated with ethnicity.
There are also within-region and racial differences in disease incidence as well as cancer characteristics owing to differences in genetics and environmental exposures (for example, higher proportion of triple-negative breast cancer, EGFR-mutated lung cancer and microsatellite instability-high colorectal cancer in certain groups).
Third, health systems research is highly context-specific, as resources, infrastructure and sociocultural values vary widely between HICs and LMICs and even within countries.
Finally, the high costs of many interventions developed in HICs render them non-implementable in LMICs.
In addition to reducing cancer-specific mortality, cancer research also brings other benefits …
… such as improved quality of clinical care, recruitment and retention of highly qualified cancer professionals and development of an effective learning cancer system.
Hence, it is imperative that LMICs conduct their own research …
… to address local and regional problems with solutions that are acceptable, feasible, effective and implementable in their respective countries and could also impact cancer control globally.
In this perspective, we discuss
- the existing situation,
- the gaps and
- possible solutions
to address these important problems and suggest key priorities in cancer research in LMICs for the next decade (Box 1).
BOX 1:
5 KEY RESEARCH PRIORITIES FOR LMICS OVER THE NEXT DECADE
1.Reduce the burden of patients presenting with advanced-stage disease via context-specific strategies at the individual, health system and population level.
This includes health promotion and awareness, primary prevention strategies, early detection and context-appropriate screening.
These should all take into account local resources, health systems and processes; economic realities; and patient, community and societal values and norms.
2.Improve access, afordability and outcomes in cancer care via solution-oriented research.
Focus on overcoming geographic, financial, sociocultural, human resource and health system barriers to accessing cancer care; economic and health policy research to inform financing strategies for cancer care; evaluation of diagnostics and treatments, including efficacy and acceptability in local populations; and scale-up of clinical trials initiated in LMICs that are focused on local priorities and with pragmatic endpoints to maximize participation and real-world applicability.
3.Emphasize country-level health economic assessment of cancer interventions and technologies, health financing mechanisms and value-based care.
Health outcomes should be measured against the cost of delivering care at the patient, system and societal level.
There is also a need to develop research methodologies and questions that are relevant to low-resource contexts and reflect the values, preferences and economic realities faced in LMICs.
4.Scale-up quality improvement and implementation research in cancer control.
This represents a high-potential area in which research, quality-improvement tools and processes, and the development of locally relevant knowledge-translation approaches could significantly improve cancer outcomes within existing resourcing and systems.
5.Leverage technology to improve cancer control supported by robust scientifc evidence.
Technology-enabled research and innovation should address major challenges in cancer care in LMICs including
- point-of-care diagnostics (lab-on-a-chip),
- telemedicine solutions (including telepathology and teleradiology),
- image analysis and pattern recognition for pathology and radiology,
- virtual reality for training and
- digital applications for collection of cancer indicators and patient-reported outcomes.

Research gaps in LMICs
Several gaps in the research enterprise of LMICs need to be addressed to promote regionally relevant research. These include :
- the scarcity of reliable data,
- a paucity of clinical trials and
- a lack of an environment conducive to research in academic institutions, including research infrastructure, trained human resources, protected time and funding.
A more detailed assessment of these gaps can help identify solutions to address them.
1.Cancer data and registries
National and subnational cancer data are a mandatory requirement for assessing the magnitude of cancer burden and an essential yardstick to evaluate efficacy or otherwise of any intervention in primary, secondary or tertiary care.
Site-specific cancer incidence, mortality and stage are poorly characterized or absent in many LMICs, with only one in five countries able to report cancer data of sufficient quality to determine incidence estimates.
Even basic data about patients diagnosed with cancer can provide valuable information to guide policy.
Sources of basic cancer data include civil registration and vital statistics systems, which register deaths from all causes and assign a cause of death, and population- and hospital-based cancer registries.
Only about two-thirds of the approximately 55 million annual total deaths worldwide are registered through a civil registration and vital statistics system, and up to half of these are not assigned a cause of death.
Coverage with population-based cancer registries (PBCRs) remains low in South America (19% of the total population is covered by a registry), Asia (15%) and Africa (13%), and sampling is predominantly sub-national, urban biased and of variable quality.
One study found that, among 190 countries, 50 (26%) did not have any cancer registry, 99 (52%) had PBCRs and only 81 (43%) had national coverage.
While 88% of HICs and 49% of upper middle-income countries had PBCRs, only 32% of LMICs and 24% of low-income countries had PBCR data; national coverage of registries was 70%, 44%, 26% and 17% in HICs, upper middle-income, lower middle-income and low-income countries, respectively.
National and subnational cancer data are a mandatory requirement for assessing the magnitude of cancer burden and an essential yardstick to evaluate efficacy or otherwise of any intervention in primary, secondary or tertiary care.
Only about two-thirds of the approximately 55 million annual total deaths worldwide are registered through a civil registration and vital statistics system, and up to half of these are not assigned a cause of death.
Coverage with population-based cancer registries (PBCRs) remains low in South America (19% of the total population is covered by a registry)
Information on stage at diagnosis and follow-up data on outcomes and long-term survival after a cancer diagnosis are less likely to be reliably captured or reported in LMICs.
The International Agency for Research on Cancer (IARC) established the Global Initiative for Cancer Registry Development (GICR) in 2011 in partnership with other national and international organizations.
The GICR has established six regional hubs and IARC collaborating centers to work with countries in supporting their registries and to provide training and research opportunities that increase the number of high-quality cancer registries globally.
Creating reliable data sources such as nationally representative PBCRs should be prioritized by all countries to guide their cancer-control plans and research priorities.
In addition, because many LMICs do not have existing cancer registries, they represent a fertile ground for innovation through application of new digital tools such as cloud-based solutions, electronic data capture, artificial intelligence and machine learning tools for quality control, new methods of data linking and engaging front-line professionals and patients in cancer registration processes.
For example, Registry Plus ( https://www.cdc.gov/cancer/npcr/tools/registryplus/) is a suite of free software programs for collection and processing of cancer registry data and is made available by the Center for Disease Control to implement the National Program of Cancer Registries in the US.
Information on stage at diagnosis and follow-up data on outcomes and long-term survival after a cancer diagnosis are less likely to be reliably captured or reported in LMICs.
In addition, because many LMICs do not have existing cancer registries, they represent a fertile ground for innovation through application of new digital tools such as
cloud-based solutions, electronic data capture, artificial intelligence and machine learning tools for quality control, new methods of data linking and engaging front-line professionals and patients in cancer registration processes.
2.Cancer clinical trials in LMICs
Cancer clinical trials of investigational new drugs remain disproportionately concentrated in HICs, align poorly with the global burden of cancer and are focused on interventions that provide small absolute gains to highly select groups of patients.
Racial and ethnic minority populations are under-represented in HIC cancer trials, further reducing generalizability to other regions.
Less than a third of registered clinical trials for the three highest-burden cancers (cervical, breast and lung) recruited patients from LMICs in 2010–2017, and many countries in sub-Saharan Africa recorded no cancer clinical trial activity.
LMIC-led phase 3 trials of anti-cancer therapies accounted for only 8% of global trials between 2014 and 2017 (ref. ).
Yet, randomized controlled trials (RCTs) from LMICs were more likely to identify effective therapies and have a larger effect size than those from HICs, suggesting important scientific contributions with real-world impact.
Ironically, even though some industry-sponsored trials evaluating new drugs do recruit patients from LMICs, most of these therapies remain inaccessible for patients due to exorbitant costs.
Cancer clinical trials of investigational new drugs remain disproportionately concentrated in HICs, align poorly with the global burden of cancer and are focused on interventions that provide small absolute gains to highly select groups of patients.
Racial and ethnic minority populations are under-represented in HIC cancer trials, further reducing generalizability to other regions.
Yet, randomized controlled trials (RCTs) from LMICs were more likely to identify effective therapies and have a larger effect size than those from HICs
Ironically, … most of these therapies remain inaccessible for patients due to exorbitant costs.
The paucity of clinical trials in regions with the greatest global burden of cancer contributes to a lack of context-specific high-quality evidence on which to base treatment decisions, clinical guidelines and resource allocation.
It also widens global disparities in cancer care by concentrating cancer knowledge generation, application, and infrastructure within HICs.
High-quality evidence generated in cancer trials in one setting does not necessarily translate across populations, subgroups, geographies or health systems.
For example, efficacy and toxicity data for chemotherapy and immunotherapy differ among ethnic groups.
All countries should be able to participate in cancer research relevant to their own populations; trial recruitment must be representative across age, sex, ethnicity, geography and socioeconomic strata.
Cancer genomics is transforming the diagnosis and treatment of cancer — but, as with clinical trials, most of this new knowledge is drawn from high-income, predominantly European-ancestry populations and threatens to perpetuate the bias against LMICs.
Development of biobanks and cancer genomics research in all parts of the world is critical to advancing knowledge and to developing precision medicine approaches that are relevant to all and do not perpetuate inequities.
Cancer genomics is transforming the diagnosis and treatment of cancer — but, as with clinical trials, most of this new knowledge is drawn from high-income, predominantly European-ancestry populations and threatens to perpetuate the bias against LMICs.
Development of biobanks and cancer genomics research in all parts of the world is critical
3.Strengthening research capacity
Over the past 3–4 decades, most HICs have developed excellent research capacity and infrastructure with trained and experienced researchers, clinical trial units (CTUs), research managers and biostatistical support.
This is lacking in LMICs, with the exception of a few centers of excellence. Efforts to strengthen research capacity in LMICs should be expanded not only at the individual level but also at the systems level.
For example, the UK Department for International Development addresses capacity strengthening at individual, organizational and institutional levels.
Working on all three levels is more likely to yield long-term results. The move from the individual to the organizational and institutional level, although more difficult, must be pursued to achieve long-term benefits and sustainability.
Strengthening at each of these levels includes capacity assessment, strategizing and planning, implementation, monitoring and evaluation.
Over the past 3–4 decades, most HICs have developed excellent research capacity and infrastructure with trained and experienced researchers, clinical trial units (CTUs), research managers and biostatistical support.
This is lacking in LMICs, with the exception of a few centers of excellence.
Efforts to strengthen research capacity in LMICs should be expanded not only at the individual level but also at the systems level.
For example, the UK Department for International Development addresses capacity strengthening at individual, organizational and institutional levels.
Strengthening at each of these levels includes capacity assessment, strategizing and planning, implementation, monitoring and evaluation
Strengthening research capacity agenda
- Individual level
- Organizational level
- Cancer Research Network
- Policy level
Individual level
For the most part, clinicians and researchers in LMICs lack formal training in research methods and are ill-equipped to conduct clinical and translational cancer research.
Therefore, enhancing knowledge and skills is a crucial element of strengthening global research capacity.
But programs designed to accomplish this need to be adapted to the local context.
For example, many LMICs have a limited number of health professionals treating cancer, and programs requiring 1 or 2 dedicated years of training in a different organization may not be feasible.
Shorter-duration courses on clinical research methodology and protocol-development workshops offer early-career researchers opportunities to learn research methods.
Continued mentoring from experienced investigators in their own institutions supported by online virtual mentoring by external experts could give early-career researchers the skills needed to carry out independent research.
However, future longer-term emphasis should be on formal research training, which provides a deeper and more thorough understanding of research methods.
The Fogarty International Center of the National Institutes of Health offers excellent opportunities for early-career researchers in LMICs.
The Collaboration for Research methods Development in Oncology (CReDO) workshop and the collaborative research network created by the National Cancer Grid (NCG) (India) are examples of how capacity building have resulted in improved research outputs ( https://tmc.gov.in/ncg).
Developing a strong cadre of clinician-scientists can enhance interdisciplinary approaches.
Once trained, these investigators should have protected time for research to best use their knowledge and capabilities, and to train the next generation of researchers.
Incentives should be provided for such activities, such as accelerated career progression, and adequate funding.
Finally, the system (salary support, infrastructure and protected time) should be attractive enough to promote sustained and meaningful academic careers for these researchers in their home countries.
For the most part, clinicians and researchers in LMICs lack formal training in research methods and are ill-equipped to conduct clinical and translational cancer research.
Therefore, enhancing knowledge and skills is a crucial element of strengthening global research capacity.
But programs designed to accomplish this need to be adapted to the local context.
Organizational level
Many LMICs do not prioritize research sufficiently to establish adequate infrastructure to support institutional researchers.
The Clinical Research Secretariat and the Department of Atomic Energy Clinical Trials Centre established in 1997 at the Tata Memorial Centre in India are examples of how creating the right infrastructural support can provide an environment for high-quality research.
Several globally practice-changing cancer clinical trials have resulted from this organizational initiative, which also conducts periodic training in research methods and good clinical practice for investigators.
Creating CTUs, academic contract research organizations and institutional ethics committees or review boards facilitates the conduct of research by support for study design, biostatistics, data management, regulatory submissions and approvals, contracts and trial insurance.
The key components of a comprehensive CTU include clinicians with wide experience in conducting clinical trials, biostatisticians, trial- and study-management teams (clinical research coordinators, study monitors, clinical project managers and data managers), database-management systems (personnel and software) and administrative staff.
A robust ethics and regulatory framework is crucial to ensure good clinical research practices and high-quality research conduct; establishing this well ahead of embarking on research is important.
Organizational support should also include curation of core facilities, datasets, biobanks and other resources that benefit multiple investigators, in particular, those at early-career stages.
Many LMICs do not prioritize research sufficiently to establish adequate infrastructure to support institutional researchers.
Creating CTUs, academic contract research organizations and institutional ethics committees or review boards facilitates the conduct of research by support for study design, biostatistics, data management, regulatory submissions and approvals, contracts and trial insurance.
The Clinical Research Secretariat and the Department of Atomic Energy Clinical Trials Centre established in 1997 at the Tata Memorial Centre in India are good examples …
Another opportunity to improve the quality of research is participation in pharma-sponsored research.
Often, in LMICs, this offers early-career researchers the chance to learn research methods while participating in clinical trials; but, for this to be truly effective, LMIC researchers should be involved in the development of clinical trial protocols including study designs and interventions, and their participation should be reflected in authorship of publications when appropriate.
Overall, the creation of, and sustained investment into, research infrastructure is essential for institutions committed to academic clinical research.
Another opportunity to improve the quality of research is participation in pharma-sponsored research.
Cancer research networks
Collaborations and networks are key to promoting research in LMICs. Most collaborations in LMICs have been with HICs, resulting in inequitable involvement and credit.
Often, LMIC researchers are merely implementers of the research with marginal involvement in its design.
Leading global health researchers have issued a ‘dire request to the global health community to challenge the ingrained and detrimental status quo that is a heritage of the colonial mindset’.
North-south collaborations can work well, provided all stakeholders work in a fair and cooperative manner.
Researchers have identified eight criteria as being important for a mutually rewarding collaboration: ‘opportunities for active involvement in cutting-edge, interesting science; effective leadership; competence of potential partners in, and commitment to good scientific practice; capacity building; respect for the needs, interests and agendas of partners; opportunities for discussion and disagreement; trust and confidence; and, justice and fairness in collaboration’.
Collaborations and networks are key to promoting research in LMICs. Most collaborations in LMICs have been with HICs, resulting in inequitable involvement and credit.
Often, LMIC researchers are merely implementers of the research with marginal involvement in its design.
Researchers have identified eight criteria as being important for a mutually rewarding collaboration …
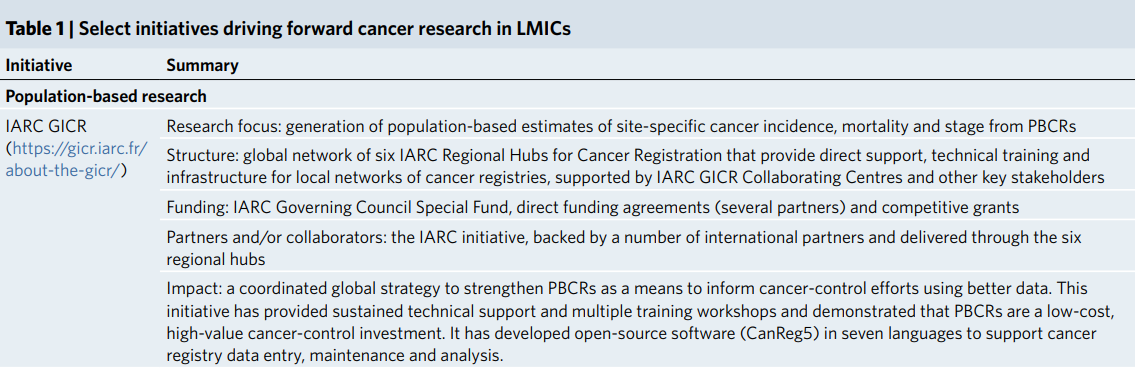
South-south collaborations and national and regional research networks are also essential to address common public health problems (Table 1).
Successful examples of these include the NCG of India ( https://tmc.gov.in/ncg), the African Organisation for Research and Training in Cancer ( https://aortic-africa.org/), the African Research Group for Oncology (ARGO) ( https://www.argo-research.org/) and the Research for Health in Conflict Middle East and North Africa (R4HC MENA) ( https://r4hc-mena.org/).
These consortia or networks can work efficiently on shared problems and common research priorities locally and globally, build long-term and sustainable relationships and conduct large-scale cancer clinical trials and epidemiological research.
Table 1 Select initiatives driving forward cancer research in LMICs









Policy level
Governments, national research organizations and funding bodies should work in concert to promote a ‘culture’ of research and integrate science and technology strategy at a political level.
Promoting leadership in research, providing incentives for high-quality research and adequate funding opportunities are necessary to motivate researchers in LMICs.
Public involvement in research is considerably less in LMICs, possibly due to lack of awareness about the importance of biomedical research.
Inadequate funding for research is a major obstacle in LMICs; this could be provided by increasing government expenditure on research, philanthropic support, tax incentives for donations and public-private partnership.
Several international organizations such as the US National Cancer Institute (NCI), the National Institute for Health Research (NIHR) and the Global Alliance for Chronic Diseases offer opportunities for funding cancer research in LMICs.
However, while HICs can play a role by offering grants for research in LMICs, this is often not a long-term and sustainable option.
Most LMICs currently spend less than 0.5% gross domestic product on research and development.
LMIC governments should realize the importance of local research and increase funding for health research in general and cancer research in particular.
These funding opportunities should also be disseminated widely within the clinician and researcher community to expand the researcher base.
Governments, national research organizations and funding bodies should work in concert to promote a ‘culture’ of research and integrate science and technology strategy at a political level.
Inadequate funding for research is a major obstacle in LMICs; this could be provided by increasing government expenditure on research, philanthropic support, tax incentives for donations and public-private partnership.
Several international organizations …offer opportunities for funding cancer research in LMICs.
However, while HICs can play a role by offering grants for research in LMICs, this is often not a long-term and sustainable option.
There is a strong economic argument supporting cancer research; every million dollars spent on cancer research in the US are estimated to produce value worth 28 million dollars in the following 50 years.
In the UK, every pound invested in cancer research generated 0.4 £ per year thereafter.
Cancer research therefore not only makes discoveries leading to improved health but also represents a good return on investment. Long-term commitment to research involving the government, academic and research institutions, civil society and the private sector is necessary.
There is a strong economic argument supporting cancer research; every million dollars spent on cancer research in the US are estimated to produce value worth 28 million dollars in the following 50 years.
In the UK, every pound invested in cancer research generated 0.4 £ per year thereafter.

What is needed in the next decade?
Radical rethinking is needed about priorities for cancer research in LMICs.
Radical rethinking is needed about priorities for cancer research in LMICs.
Leading cancer centers in HICs focus much of their research on what is considered ‘state of the art’, including precision medicine, immunotherapy, next-generation sequencing, robotic surgery and proton therapy among others; most of these approaches would have low priority in LMICs.
The burning issues in cancer care in LMICs are late-stage presentation, barriers of access and affordability, and variable quality of care; cancer research in LMICs needs to be aligned with these problems.
LMICs should focus their research on cancers that are common or unique to their region with emphasis on implementable solutions: for example, oral and nasopharyngeal cancer in parts of Asia; hepatobiliary cancers in parts of India, Thailand, Mongolia, Chile and Egypt; and prostate, breast and cervical cancers and Kaposi’s sarcoma in Africa.
Research in LMICs should place emphasis on the best-value interventions, based on local capacity.
Leading cancer centers in HICs focus much of their research on what is considered ‘state of the art’, including precision medicine, immunotherapy, next-generation sequencing, robotic surgery and proton therapy among others;
most of these approaches would have low priority in LMICs.
LMICs should focus their research on cancers that are common or unique to their region with emphasis on implementable solutions…
Research in LMICs should place emphasis on the best-value interventions, based on local capacity.
In Box 1 and the next sections, we describe our top five priorities for cancer research in LMICs.
BOX 1 (Short Version — See above for the complete box)
5 KEY RESEARCH PRIORITIES FOR LMICS OVER THE NEXT DECADE
1.Reduce the burden of patients presenting with advanced-stage disease via context-specific strategies at the individual, health system and population level.
2.Improve access, affordability and outcomes in cancer care via solution-oriented research.
3.Emphasize country-level health economic assessment of cancer interventions and technologies, health financing mechanisms and value-based care.
4.Scale-up quality improvement and implementation research in cancer control.
5.Leverage technology to improve cancer control supported by robust scientifc evidence.
1.Reduction in burden of patients diagnosed with advanced-stage cancers
The overall incidence of cancers in LMICs is growing due to increased life expectancy, expanding urbanization and lifestyle changes.
In addition, the stage distribution of patients with cancer is heavily skewed towards advanced disease.
The overall incidence of cancers in LMICs is growing due to increased life expectancy, expanding urbanization and lifestyle changes.
In addition, the stage distribution of patients with cancer is heavily skewed towards advanced disease.
This is due to lack of awareness among the general public and primary-care physicians, lack of access to diagnostic facilities including imaging and pathology, and geographic distances from hospitals offering comprehensive cancer services.
Moreover, conventional screening methods such as mammography, human papillomavirus DNA testing and Pap smears are difficult to implement due to inadequate resources and expertise.
Research in LMICs should focus on primary prevention and interventions to reduce risk factors including infection control, tobacco cessation and other lifestyle changes.
Research on how best to increase public awareness, educate primary health practitioners, and combat stigma and secrecy associated with cancer diagnosis should be emphasized to reduce diagnostic delays.
Research in LMICs should focus on primary prevention and interventions to reduce risk factors including infection control, tobacco cessation and other lifestyle changes.
Research on how best to increase public awareness, educate primary health practitioners, and combat stigma and secrecy associated with cancer diagnosis should be emphasized to reduce diagnostic delays.
There is a need for better understanding of health behavior and sociocultural norms that influence early presentation and participation in cancer screening.
Screening techniques that can be implementable in low-resource settings include inspection of the cervix with acetic acid (VIA) and clinical breast examination by trained health workers, shown to reduce cervical and breast cancer mortality, respectively, in a large cluster randomized trial in India.
The screening tool used should also be demonstrated not to result in overdiagnosis, especially when the cancer care delivery is inadequate to deal with the excess burden.
There is a need for better understanding of health behavior and sociocultural norms that influence early presentation and participation in cancer screening. e.g. … inspection of the cervix with acetic acid (VIA) …
The screening tool used should also be demonstrated not to result in overdiagnosis…
Development and evaluation of point-of-care devices or tests for diagnosis or screening such as self-sampling for cervical cancer and the use of mobile (smartphone) technology for image transmission and artificial intelligence for interpretation are exciting new areas of research.
However, new technologies should undergo rigorous testing in randomized trials along with implementation research before widespread adoption.
Development and evaluation of point-of-care devices or tests for diagnosis or screening such as self-sampling for cervical cancer and the use of mobile (smartphone) … are exciting new areas of research.
However, new technologies should undergo rigorous testing in randomized trials along with implementation research before widespread adoption.
2.Research on improving access to and affordability and outcomes of cancer care
Cancer treatment outcomes are worse in LMICs than in HICs, even when adjusted for disease stage.
Probable reasons include barriers to access, high cost and lower quality of care.
Research to overcome these barriers with innovative solutions should be prioritized.
Cancer treatment outcomes are worse in LMICs than in HICs, even when adjusted for disease stage. Probable reasons include barriers to access, high cost and lower quality of care.
Research to overcome these barriers with innovative solutions should be prioritized.
Costs of cancer care are burgeoning globally, but they affect LMICs disproportionately.
With limited public health care expenditure, the cost of medical care falls on out-of-pocket expenses from individuals; this limits access to optimal treatment and contributes to health care-associated impoverishment.
Costs of cancer care are burgeoning globally, but they affect LMICs disproportionately.
Development of generic and biosimilar drugs that are cheaper and improve access requires substantial investment and active participation from industry, in place of the frequent opposition from those pharmaceutical companies that hold original patents.
Alternative strategies include research on repurposed drugs, metronomic chemotherapy, alternative dosing strategies, hypofractionated radiation regimens and radioisotope-tagged targeted therapy; these have the potential to bring costs down without adversely impacting outcomes.
Alternative strategies include research on repurposed drugs, metronomic chemotherapy, alternative dosing strategies, hypofractionated radiation regimens and radioisotope-tagged targeted therapy; …
For example, early studies of immunotherapy agents such as pembrolizumab and nivolumab showed no dose response above a particular (low) dose and target occupancy lasting for months.
These agents are highly effective in improving survival with several types of cancer, but the World Health Organization (WHO) has not included them in their essential medicines list (with the exception of malignant melanoma) because their high cost renders then inaccessible to most people in LMICs.
Research leading to the development of these agents was supported by public funding and rewarded with a Nobel prize; therefore, their lack of availability is an affront to international ethics.
It is quite possible that these drugs (and other targeted agents) could be given at much lower doses than those currently approved and much less often.
Trials comparing doses approved in HICs with much lower doses could greatly improve access in LMICs, but these trials are unlikely to be carried out by the pharmaceutical industry.
In one example, a simple strategy of administering low-dose abiraterone after a low-fat meal showed near equivalence with standard dosing in advanced prostate cancer and is an example of reducing cost by 75% without loss of activity.
For immuno-oncology agents, this cost reduction could be even greater.
Despite financial toxicity and imperfect access to optimal treatment being a problem everywhere, these are typically not priority areas for research in HICs, and LMICs could lead the way in these types of research.
Similarly, surgical, radiation and palliative care research are under-represented in HIC research and should be prioritized in LMICs.
LMICs could lead the way in some types of research, such as financial toxicity and imperfect access to optimal treatment
Similarly, surgical, radiation and palliative care research are under-represented in HIC research and should be prioritized in LMICs.
3.Value-based care and health economics
The rapidly growing expenditure in most cancer health systems is on new medicines.
While some new therapies have been transformational for patient outcomes, most of these offer very small benefits.
The median gain in survival among new cancer medicines is 2–3 months.
Growing evidence supports the concept of a substantial efficacy-effectiveness gap in which the modest gains observed in RCTs are almost certainly even smaller in routine practice. These modest gains need to be balanced against side effects and the costs of care.
The rapidly growing expenditure in most cancer health systems is on new medicines.
While some new therapies have been transformational for patient outcomes, most of these offer very small benefits.
These issues pose fundamental challenges to sustainable and impactful cancer care in all health systems but are most acute in lower-resource settings.
Adoption and diffusion of cancer technology (diagnostics and therapeutics especially) is often based on weak evidence internationally and a paucity of country-specific data and evaluation.
This is problematic in all countries, but, in LMICs with comparatively fewer public health resources and many competing health and societal needs, appraisal of cancer technologies before their adoption is critical.
… in LMICs with … appraisal of cancer technologies before their adoption is critical.
There is a dearth of literature on the macroeconomic and microeconomic impacts of cancer, the cost effectiveness of cancer-control interventions in LMICs and the broader economic, welfare and social value of investing in cancer care.
Yet many of the implementation barriers in these countries are related to costs and how public health care expenditure is planned.
Moreover, these problems are country specific (costs vary substantially between and even within countries), and this knowledge is critical to guiding sound investments in cancer control.
Only 8–14% of published economic evaluations of health interventions are from LMICs.
Furthermore, many methods used in health economic analyses fail to account for the different economic contexts, health impacts, individual and societal preferences or values of LMICs.
For example, disability weights in cost-effectiveness analyses are drawn from high-income populations who may experience the impact of an illness very differently than someone in a resource-poor setting, and the social discount rate generally applied in global health and cancer economic analyses of 3% annually is inconsistent with the higher rates of economic growth in LMICs, thereby overvaluing future costs and health benefits of interventions.
Macroeconomic analyses in health and cancer focus on productivity gains and losses measured through changes in gross domestic product, which fail to capture the monetary and non-monetary impacts of illness on individuals or society and reflect Western values.
… many methods used in health economic analyses fail to account for the different economic contexts, health impacts, individual and societal preferences or values of LMICs.
4.Quality improvement and implementation research
Survival rates of cancer are strikingly lower in LMICs than in HICs.
While this may be due partly to more non-lethal cancers being identified by population-level screening in HICs, the more substantive reason is the variation in the quality of care provided in LMICs.
Survival rates of cancer are strikingly lower in LMICs than in HICs.
While this may be due partly to more non-lethal cancers being identified by population-level screening in HICs, the more substantive reason is the variation in the quality of care provided in LMICs.
Research evaluating the quality of care and a robust program in quality improvement combined with quality assurance and quality control is necessary.
Examples include safe chemotherapy and initiatives to decrease medication-administration errors, reducing diagnostic delays and wait times for cancer treatment, and rational antibiotic use to prevent antimicrobial resistance.
Research in this area would involve a systematic plan-do-study-act cycle framework using standard quality-improvement tools such as the Pareto diagram or the fishbone diagram, with outcome metrics being measured.
Research evaluating the quality of care and a robust program in quality improvement combined with quality assurance and quality control is necessary.
There is failure to implement many interventions that are proven to be cost effective, even in HICs.
In the US and Europe, 30–50% of patients do not receive optimal evidence-based clinical interventions, and this number is likely much higher in LMICs.
Implementation gaps are an important cause of failure of health policies and reforms such as decentralization of care delivery, health care regulation and improving primary health care in LMICs.
There is failure to implement many interventions that are proven to be cost effective, even in HICs.
In the US and Europe, 30–50% of patients do not receive optimal evidence-based clinical interventions, and this number is likely much higher in LMICs.
One potential strategy involves a network of providers with defined complexity tiers (hub-and-spoke model of care) safely decentralizing treatment of common cancers with relatively straightforward care requirements, but this involves substantial reorganization of the health care infrastructure.
However, evaluating the efficacy of new technology-enabled solutions such as telementoring, telepathology, remote monitoring of patient-reported outcomes, expert online opinions and virtual multidisciplinary tumor boards could help bridge the implementation gap in the short to medium term and could better support the hub-and-spoke model.
One potential strategy involves a network of providers with defined complexity tiers (hub-and-spoke model of care) safely decentralizing treatment of common cancers …
However, evaluating the efficacy of new technology-enabled solutions … could help bridge the implementation gap in the short to medium term and could better support the hub-and-spoke model.
PBCRs, in addition to providing information about case mix and incidence, can become an even more powerful tool when they are linked to treatment records facilitating research on delivery of health services and health system performance.
PBCRs, in addition to providing information about case mix and incidence, can become an even more powerful tool when they are linked to treatment records …
Overall, well-conducted implementation research is lacking in LMICs.
Generating context-specific data on access to care, quality of care and outcomes is a crucial step to close gaps between evidence and practice and can yield immediate benefits at the population level.
Overall, well-conducted implementation research is lacking in LMICs. Generating context-specific data on access to care, quality of care and outcomes is a crucial step to close gaps between evidence and practice and can yield immediate benefits at the population level.
Successful examples of effective implementation include the directly observed treatment, short-course strategy recommended by the WHO to improve compliance with tuberculosis treatment and the use of patient navigators (Kevats, at the Tata Memorial Centre) to help patients through their care pathway.
Other examples include the increasing use of mobile and internet technology (m-Health and e-health).
Priority areas for implementation research include evaluation of a decentralized network model of cancer care and the expanding role of primary care in cancer control (cancer diagnosis, survivorship and supportive or palliative care).
Integrating this into the broader scope of health services research and involving policy makers in the research team is important.
Priority areas for implementation research include evaluation of a decentralized network model of cancer care and the expanding role of primary care in cancer control (cancer diagnosis, survivorship and supportive or palliative care).
Considering the relatively near-term impact of implementing cost-effective, evidence-based interventions in the real-world setting, health services and systems research should be prioritized in LMICs.
With the escalating cost of cancer care, HICs are also focusing on implementation science.
However, entrenched practices are difficult to change.
LMICs, with their emerging health systems, have an advantage to be able to lead the development of more efficient modern processes and practices and in turn impact HIC systems.
Considering the relatively near-term impact of implementing cost-effective, evidence-based interventions in the real-world setting, health services and systems research should be prioritized in LMICs.
LMICs, with their emerging health systems, have an advantage to be able to lead the development of more efficient modern processes and practices and in turn impact HIC systems
5.Leveraging technology to improve cancer control
Technology has advanced considerably in the fields of molecular biology, next-generation sequencing, precision medicine, robotics, advanced imaging and radiation, to name but a few.
However, for the most part, these advances are not directly applicable to patients in LMICs over the near term.
Technology has advanced considerably in the fields of molecular biology, next-generation sequencing, precision medicine, robotics, advanced imaging and radiation, to name but a few.
However, for the most part, these advances are not directly applicable to patients in LMICs over the near term.
Less studied is the intersection between technology and medicine in addressing pressing health care (including cancer) problems in LMICs.
Interdisciplinary research involving physicians, researchers, medicinal chemists, scientists, engineers, experts in digital health, artificial intelligence and machine learning has the potential to identify problems and find solutions that are well suited to LMIC contexts.
Such collaboration could address the four thematic areas of research described earlier.
Less studied is the intersection between technology and medicine in addressing pressing health care (including cancer) problems in LMICs.
Examples of technology-enabled research that could address real-world problems in LMICs include
- point-of-care diagnostics (lab-on-a-chip),
- telemedicine solutions (including telepathology and teleradiology),
- image analysis and pattern recognition for pathology and radiology,
- virtual reality in training,
- patient care pathways and
- digital applications for patient-reported outcomes, among others.
Examples of technology-enabled research that could address real-world problems in LMICs … are abundant …
However, these new, intuitively appealing technologies must be validated in real-life situations.
However, these new, intuitively appealing technologies must be validated in real-life situations.
Previous instances of clinical decision-support systems that promised more than they delivered illustrate how technology can overtake the scientific method.
Similarly, several digital applications have been launched without appropriate research to validate their performance.
The Affordable Cancer Technologies program of the NCI is a good example of focusing efforts on technology-enabled solutions along with validation in LMICs ( https://www.cancer.gov/about-nci/organization/cgh/research-training-programs/affordable-cancer-technology).
With the caveat of requiring rigorous evaluation, judicious use of technology has the potential to enable LMICs to achieve much-improved health outcomes.

Summary
Global cancer research has thus far been driven primarily by HICs, which have different cancer statistics, research priorities, capacity, infrastructure and health systems than LMICs.
Adopting HIC research findings in LMICs is likely to yield suboptimal outcomes, and there is a need to urgently scale up locally relevant cancer research in these countries.
Strengthening research capacity at individual, organizational, network and policy levels is important for long-term benefit and sustainability.
LMICs have an excellent opportunity to address important questions in cancer research that could impact cancer control globally.
Governments, policy makers, funding agencies, health care organizations and leaders, researchers and the public should work together and show strong commitment to promote cancer research in LMICs.
Cite this article
Pramesh, C.S., Badwe, R.A., Bhoo-Pathy, N. et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med 28, 649–657 (2022). https://doi.org/10.1038/s41591-022-01738-x
Originally published at https://www.nature.com on April 19, 2022.
About the authors
C. S. Pramesh 1 ✉, Rajendra A. Badwe1 , Nirmala Bhoo-Pathy2 , Christopher M. Booth3 , Girish Chinnaswamy1 , Anna J. Dare4, Victor Piana de Andrade5 , David J. Hunter6,7, Satish Gopal8, Mary Gospodarowicz9 , Sanjeeva Gunasekera10, Andre Ilbawi11, Sharon Kapambwe12, Peter Kingham13, Tezer Kutluk14, Nirmal Lamichhane15, Miriam Mutebi16, Jackson Orem17, Groesbeck Parham18, Priya Ranganathan1 , Manju Sengar1 , Richard Sullivan 19, Soumya Swaminathan11, Ian F. Tannock9 , Vivek Tomar20, Verna Vanderpuye21, Cherian Varghese11 and Elisabete Weiderpass 22
Authors affiliations
1 Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, India.
2 Centre for Epidemiology and Evidence-Based Practice, University of Malaya, Kuala Lumpur, Malaysia. ]
3 Departments of Oncology and Public Health Sciences, Queen’s University, Kingston, Ontario, Canada.
4Department of Surgery, University of Toronto, Toronto, Ontario, Canada.
5 A.C. Camargo Cancer Center, São Paulo, Brazil.
6 Nuffield Department of Population Health, University of Oxford, Oxford, UK.
7 Harvard T.H. Chan School of Public Health, Boston, MA, USA.
8 Centre for Global Health, National Cancer Institute, Rockville, MD, USA.
9 Princess Margaret Cancer Centre and University of Toronto, Toronto, Ontario, Canada.
10 National Cancer Institute Sri Lanka, Colombo, Sri Lanka.
11 World Health Organization, Geneva, Switzerland.
12 World Health Organization Africa, Harare, Zimbabwe.
13 Memorial Sloan Kettering Cancer Center, New York, NY, USA.
14 Faculty of Medicine and Cancer Institute, Hacettepe University, Ankara, Turkey.
15 B.P. Koirala Memorial Cancer Hospital, Bharatpur, Nepal.
16 Aga Khan University, Nairobi, Kenya. ]
17 Uganda Cancer Institute, Kampala, Uganda.
18 University of North Carolina, Chapel Hill, NC, USA.
19 Institute of Cancer Policy, King’s College London, London, UK.
20 Rise To Survive Cancer, New Delhi, India.
21 National Center for Radiotherapy Oncology and Nuclear Medicine and Korle Bu Teaching Hospital, Accra, Ghana.
22 International Association for Research on Cancer, Lyon, France
References
See original publication












