This is a republication of the article “Scientists develop blood test for Alzheimer’s disease”, with the title above.
institute for health transformation
Joaquim Cardoso MSc
January 1, 2023
Scientists say test could replace a costly brain scan or painful lumbar puncture and enable earlier detection of disease
SOURCE:
The Guardian
Linda Geddes Science correspondent
Wed 28 Dec 2022
Scientists have developed a blood test to diagnose Alzheimer’s disease without the need for expensive brain imaging or a painful lumbar puncture, where a sample of cerebrospinal fluid (CSF) is drawn from the lower back.
If validated, the test could enable faster diagnosis of the disease, meaning therapies could be initiated earlier.
Alzheimer’s is the most common form of dementia, but diagnosis remains challenging — particularly during the earlier stages of the disease.
Alzheimer’s is the most common form of dementia, but diagnosis remains challenging — particularly during the earlier stages of the disease.
Current guidelines recommend detection of three distinct markers:
- abnormal accumulations of amyloid
- and tau proteins,
- as well as neurodegeneration — the slow and progressive loss of neuronal cells in specified regions of the brain.
This can be done through a combination of brain imaging and CSF analysis.
However, a lumbar puncture can be painful and people may experience headaches or back pain after the procedure, while brain imaging is expensive and takes a long time to schedule.
However, a lumbar puncture can be painful and people may experience headaches or back pain after the procedure, while brain imaging is expensive and takes a long time to schedule.
Prof Thomas Karikari at the University of Pittsburgh, in Pennsylvania, US, who was involved in the study, said: “A lot of patients, even in the US, don’t have access to MRI and PET scanners. Accessibility is a major issue.”
“A lot of patients, even in the US, don’t have access to MRI and PET scanners. Accessibility is a major issue.”
The development of a reliable blood test would be an important step forwards.
“A blood test is cheaper, safer and easier to administer, and it can improve clinical confidence in diagnosing Alzheimer’s and selecting participants for clinical trial and disease monitoring,” Karikari said.
“A blood test is cheaper, safer and easier to administer, and it can improve clinical confidence in diagnosing Alzheimer’s and selecting participants for clinical trial and disease monitoring,
Although current blood tests can accurately detect abnormalities in amyloid and tau proteins, detecting markers of nerve cell damage that are specific to the brain has been harder.
Karikari and his colleagues around the world focused on developing an antibody-based blood test that would detect a particular form of tau protein called brain-derived tau, which is specific to Alzheimer’s disease.
They tested it in 600 patients at various stages of Alzheimer’s and found that levels of the protein correlated well with levels of tau in the CSF, and could reliably distinguish Alzheimer’s from other neurodegenerative diseases.
Protein levels also closely corresponded with the severity of amyloid plaques and tau tangles in brain tissue from people who had died with Alzheimer’s.
The research was published in the journal Brain.
The next step will be to validate the test in a broader range of patients, …
… including those from varied racial and ethnic backgrounds, and those suffering from different stages of memory loss or other potential dementia symptoms.
Karikari also hopes that monitoring levels of brain-derived tau in the blood could improve the design of clinical trials for Alzheimer’s treatments.
Karikari also hopes that monitoring levels of brain-derived tau in the blood could improve the design of clinical trials for Alzheimer’s treatments.
Originally published at https://www.theguardian.com on December 28, 2022.
REFERENCE PUBLICATION

Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration
Brain
Fernando Gonzalez-Ortiz, Michael Turton, Przemysław R Kac, Denis Smirnov, Enrico Premi, Roberta Ghidoni, Luisa Benussi, Valentina Cantoni, Claudia Saraceno, Jasmine Rivolta, Nicholas J Ashton, Barbara Borroni, Douglas Galasko, Peter Harrison, Henrik Zetterberg, Kaj Blennow, Thomas K Karikari
27 December 2022
Abstract
- Blood-based biomarkers for amyloid beta and phosphorylated tau show good diagnostic accuracies and agreements with their corresponding CSF and neuroimaging biomarkers in the amyloid / tau / neurodegeneration [A/T/(N)] framework for Alzheimer’s disease.
- However, the blood-based neurodegeneration marker neurofilament light is not specific to Alzheimer’s disease while total-tau shows lack of correlation with CSF total-tau.
- Recent studies suggest that blood total-tau originates principally from peripheral, non-brain sources.
- We sought to address this challenge by generating an anti-tau antibody that selectively binds brain-derived tau and avoids the peripherally expressed ‘big tau’ isoform.
- We applied this antibody to develop an ultrasensitive blood-based assay for brain-derived tau, and validated it in five independent cohorts (n = 609) including a blood-to-autopsy cohort, CSF biomarker-classified cohorts and memory clinic cohorts.
- In paired samples, serum and CSF brain-derived tau were significantly correlated (rho = 0.85, P < 0.0001), while serum and CSF total-tau were not (rho = 0.23, P = 0.3364).
- Blood-based brain-derived tau showed equivalent diagnostic performance as CSF total-tau and CSF brain-derived tau to separate biomarker-positive Alzheimer’s disease participants from biomarker-negative controls.
- Furthermore, plasma brain-derived tau accurately distinguished autopsy-confirmed Alzheimer’s disease from other neurodegenerative diseases (area under the curve = 86.4%) while neurofilament light did not (area under the curve = 54.3%).
- These performances were independent of the presence of concomitant pathologies.
- Plasma brain-derived tau (rho = 0.52–0.67, P = 0.003), but not neurofilament light (rho = −0.14–0.17, P = 0.501), was associated with global and regional amyloid plaque and neurofibrillary tangle counts.
- These results were further verified in two memory clinic cohorts where serum brain-derived tau differentiated Alzheimer’s disease from a range of other neurodegenerative disorders, including frontotemporal lobar degeneration and atypical parkinsonian disorders (area under the curve up to 99.6%).
- Notably, plasma/serum brain-derived tau correlated with neurofilament light only in Alzheimer’s disease but not in the other neurodegenerative diseases.
- Across cohorts, plasma/serum brain-derived tau was associated with CSF and plasma AT(N) biomarkers and cognitive function.
- Brain-derived tau is a new blood-based biomarker that outperforms plasma total-tau and, unlike neurofilament light, shows specificity to Alzheimer’s disease-type neurodegeneration.
- Thus, brain-derived tau demonstrates potential to complete the AT(N) scheme in blood, and will be useful to evaluate Alzheimer’s disease-dependent neurodegenerative processes for clinical and research purposes.
Brain-derived tau is a new blood-based biomarker that outperforms plasma total-tau and, unlike neurofilament light, shows specificity to Alzheimer’s disease-type neurodegeneration.
Thus, brain-derived tau demonstrates potential to complete the AT(N) scheme in blood, and will be useful to evaluate Alzheimer’s disease-dependent neurodegenerative processes for clinical and research purposes.
Infographic
Figure 1 –Design and characterization of the TauJ.5H3 sheep monoclonal antibody specific for CNS-derived tau isoforms
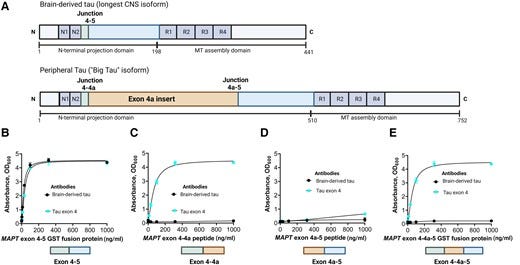
Figure 2 — Technical validation of a novel assay to measure BD-tau in blood.
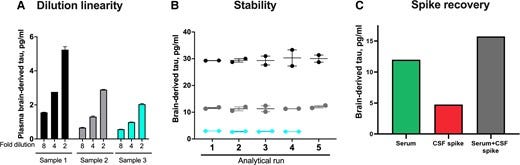
Figure 3 — Concentrations and correlation of BD-tau in paired serum and CSF samples
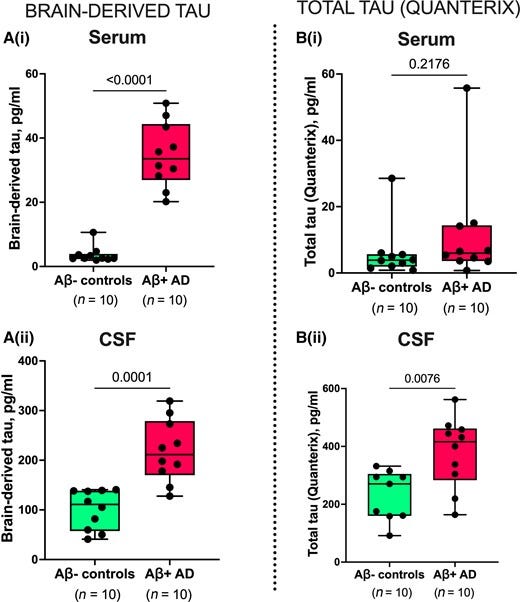
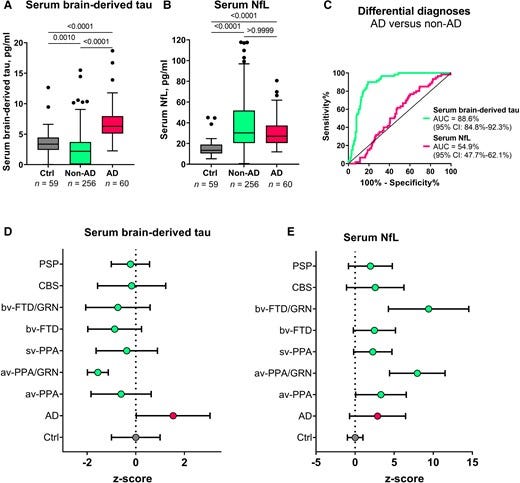
Figure 4 — Plasma BD-tau accurately differentiates autopsy-verified Alzheimer’s disease from other neurodegenerative diseases.
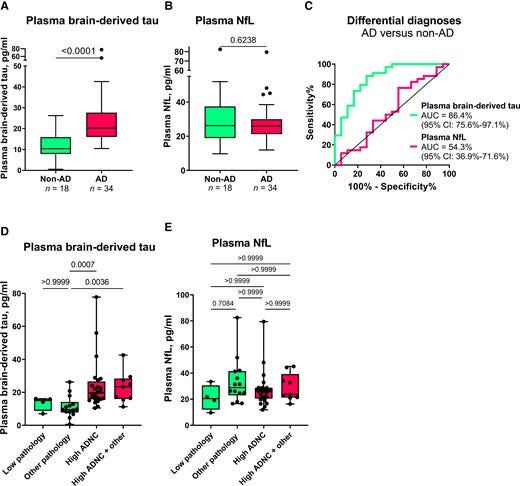
Figure 5 — Serum BD-tau profile in Alzheimer’s disease versus several other neurodegenerative diseases in Memory Clinic Cohort 1.
Introduction
The AT(N) (amyloid, tau, neurodegeneration) research framework provides a unified scheme that emphasizes on pathophysiological evidence of amyloid beta (A), tau (T) and neurodegeneration (N) for the definition and staging of Alzheimer’s disease.1–3 However, the framework currently relies on established CSF and neuroimaging biomarkers that have major challenges from economical, practical and logistical perspectives that limit their widespread applications, particularly in contexts that require cost-effective and high-throughput assessments for biological evidence of Alzheimer’s disease. For example, given the paucity of dementia specialists in many hospital systems,4 biomarker screening at the primary care level would be beneficial to streamline patient management including referrals to specialist practitioners.5–8 Moreover, as therapeutic trials are now required to show biomarker evidence of candidate drug efficacy,9 it is essential that only participants with confirmed underlying pathology are included and further monitored in the course of trials.6 The Alzheimer’s disease field will therefore benefit from biomarker modalities that have improved simplicity, accessibility, convenience and cost-effectiveness without compromising on performance.5–8
The development of AT(N) blood biomarkers improves the needed scalability for large-scale diagnostic, prognostic and therapeutic trial applications. Plasma amyloid beta (Aβ)42/Aβ40 methods, measured using immunoprecipitation-mass spectrometry methods, have shown good accuracies to detect Aβ abnormalities in Alzheimer’s disease that are absent in neurodegenerative diseases without amyloidosis.10–13 Plasma p-tau, including those targeting p-tau181, p-tau217 and p-tau231, have also demonstrated excellent diagnostic performances to detect and to differentiate Alzheimer’s disease from other neurodegenerative diseases.14–19 Plasma Aβ and p-tau therefore show promise as the A and T biomarkers, respectively, in the AT(N) framework.6,8,20 For neurodegeneration (N), however, plasma neurofilament light chain (NfL) has demonstrated excellent diagnostic performance to identify Alzheimer’s disease compared with controls, but is unable to distinguish it from other neurodegenerative diseases.21–24 For these reasons, plasma NfL may not be optimal for use as an Alzheimer’s disease-specific neurodegeneration marker. Moreover, current plasma total-tau (t-tau) assays do not show good diagnostic utility,24–29 contrary to CSF t-tau that reliably reflects neurodegeneration in Alzheimer’s disease but not in other neurodegenerative diseases like Parkinson’s disease, Lewy body dementia and frontotemporal dementia.30–34 Plasma t-tau concentrations show large overlaps between diagnostic groups and do not correlate with CSF t-tau, suggesting that plasma and CSF t-tau do not originate from the same tissue sources.25,27,29 Indeed, while tau is known to be highly abundant in the CNS, the protein is also present in peripheral tissues (e.g. liver, kidney, heart).35,36 The protein structure of tau in the CNS and PNS has fundamental differences in splice variants: while there are six tau isoforms of varying lengths in the adult human brain, the main form of tau in the PNS is distinguishable from these isoforms by the presence of a large peptide insert resulting from the transcription of an extra exon (exon 4a) of the MAPT gene.37,38 Since CSF t-tau, but not plasma t-tau, agrees with PET and neuropathological evidence of Alzheimer’s disease,30,32–34 it is plausible that the tau forms measured by the blood assay originate, to a large extent, from non-CNS sources. In line with this reasoning, a recent study estimated that only a fifth of the signal measured by plasma t-tau is brain-derived while the remainder originates from peripheral sources.25 As a result, plasma t-tau tends to show promising diagnostic function largely in disorders with acute increases in CNS tau production and release, including traumatic brain injury and acute stroke following cardiac arrest.39–44 Together, a blood-based biomarker for neurodegeneration specific to Alzheimer’s disease is currently lacking. Discovery of such a biomarker would complete the AT(N) system in blood, and enable examination of neurodegenerative process(es) specific to Alzheimer’s disease pathogenesis while differentiating these from neurodegenerative mechanisms common to related dementias.
In this study, we hypothesized that: (i) an immunoassay can be innovated to selectively measure brain-derived tau (BD-tau) in blood by using an antibody engineered to specifically target tau isoforms originating from the brain; (ii) such a novel assay would show strong correlations between plasma and CSF levels; and (iii) the assay would demonstrate specificity to Alzheimer’s disease by showing good performances to differentiate it from non-Alzheimer’s disease neurodegenerative diseases. Here, we report the development, analytical and clinical validation of a novel blood-based biomarker that is specific for BD-tau. In five independent research cohorts (n = 609 participants), we evaluated the capabilities of this new blood biomarker to: (i) differentiate neurochemically defined Alzheimer’s disease from biomarker-negative controls; (ii) distinguish Alzheimer’s disease from other neurodegenerative diseases, including in a cohort with neuropathological confirmation; and (iii) to associate with the severity of plaque and tangle pathologies at autopsy, CSF AT(N) biomarkers (including in paired plasma/serum versus CSF samples), and cognition.
Material, Methods, Results & Other Sections
See the original publication (this is an excerpt version)

Discussion
In this study, we present the development and validation of an ultrasensitive immunoassay, and report clinical performance results in five independent cohorts for an improved blood-based t-tau biomarker, BD-tau. In short, plasma BD-tau was shown to be an Alzheimer’s disease-type neurodegeneration biomarker that can discriminate between autopsy-verified Alzheimer’s disease from other neurodegenerative diseases, and in addition is associated with clinical severity of disease in the Neuropathology cohort. The significance of this biomarker, which also explains these findings, is that blood and CSF levels correlate strongly in paired samples. The assay was developed using a monoclonal antibody that selectively binds to CNS tau isoforms (hence the name BD-tau). This property makes it superior to the current plasma t-tau biomarker that does not correlate with CSF t-tau, probably because it also captures tau from peripheral sources.25,27,29 Furthermore, BD-tau in blood, like NfL, has high diagnostic accuracy to detect neurodegeneration in Alzheimer’s disease. However, plasma BD-tau demonstrated the novel finding of being able to accurately distinguish pathologically confirmed Alzheimer’s disease from several other neurodegenerative diseases while plasma NfL did not. These performances were specific to the neuropathological diagnosis of Alzheimer’s disease, and were unaffected by mixed pathologies. Furthermore, plasma BD-tau, but not plasma NfL, was associated with global and regional amyloid-plaque and NFT counts in the Neuropathology cohort. Moreover, correlations between plasma/serum BD-tau and NfL were observed only in individuals with Alzheimer’s disease but not those with other neurodegenerative diseases.
Attempts to develop a blood-based t-tau biomarker with similar performance as CSF t-tau have been challenging. Plasma t-tau does not correlate with CSF t-tau when measured in paired samples.25,27,29 In agreement, several studies have reported poor diagnostic performances of plasma t-tau for Alzheimer’s disease and for differential diagnosis of Alzheimer’s disease versus other neurodegenerative diseases.24–29,59,60 Since tau protein is expressed in several peripheral sources in addition to the CNS,35,36 we hypothesized that plasma t-tau is significantly affected by tau from peripheral sources. An estimated 80% of the blood t-tau signal originates from peripheral tissues, meaning that the remaining 20% contribution from the CNS is unlikely to result in significant overall differences even when assuming typical fold increases of two to three in Alzheimer’s disease participants.25 Plasma t-tau rather shows diagnostic and prognostic utility in acute neurological disorders where CNS-derived tau levels in blood increase exponentially over a short duration presumably due to blood–brain barrier impairment.40,42,60,61
To address this problem, we aimed to develop a novel blood biomarker that selectively recognizes tau derived from the brain and avoids tau from peripheral sources. We took advantage of the fact that the MAPT gene has multiple splice variants expressed in a tissue-dependent pattern.36,37 Tau in the adult human brain has six isoforms between 352 and 441 amino acids long.36,62 However, tau in peripheral tissues — including the liver, kidney, heart and pancreas — is predominantly of the high molecular weight (‘big tau’) isoform with the exon 4a insert (Fig. 1A).36 Big tau is preferentially localized in peripheral tissues where it is the main form of tau expressed in the adult PNS.38,63 We hypothesized that by generating an antibody specifically against the junction between exons 4 and 5, we could develop a novel immunoassay that selectively targets BD-tau in blood. Biochemical characterization of the resulting monoclonal antibody, TauJ.5H3, the sequence of which was verified by epitope mapping (data not shown) showed that it only bound to recombinant protein constructs that had the exon 4–5 junction intact, and not those that stretched over exons 4–4a, 4a–5 and 4–4a–5 (Fig. 1). In contrast, the tau exon-4 antibody recognized all constructs that included the exon 4, including those lacking the exon 4–5 junction. The ultrasensitive immunoassay we developed using the TauJ.5H3 antibody showed strong dilution linearity, within- and between-run stability, and suitability for use in both plasma and serum. Importantly, the strong correlation between BD-tau measured in serum/plasma and paired CSF samples is an indication that it targets brain-originating tau forms just like CSF t-tau and CSF BD-tau. This finding is highly significant given that several independent studies have reported that plasma t-tau does not correlate with CSF t-tau27–29 (as also demonstrated herein), which may be partly to blame for its poor diagnostic performance.
The most well-validated blood biomarker for neurodegeneration, NfL, is unable to differentiate between Alzheimer’s disease and other dementias due to its increases in a wide range of neurodegenerative disorders.21,56,64 Consequently, the dementia research field currently lacks a blood biomarker that is specifically altered as a result of Alzheimer-type neurodegenerative changes, such as how plasma p-tau is to tau phosphorylation/pathology in the AT(N) framework.6,8,65 Our findings indicate that plasma BD-tau might be a biomarker that is specific for Alzheimer’s disease-type neurodegeneration and can discriminate Alzheimer’s disease from other neurodegenerative diseases, as shown previously for CSF t-tau.30–34 This conclusion is supported by our findings that plasma BD-tau was increased to the same extent in individuals diagnosed with Alzheimer’s disease at autopsy irrespective of whether or not they had mixed pathologies (Figs. 4 and 5). In comparison, the biomarker levels were significantly lower in the non-Alzheimer group. However, plasma NfL failed to distinguish Alzheimer’s disease from other diseases, affirming its limitations for differential diagnosis.
If BD-tau in blood is an Alzheimer’s disease-specific neurodegeneration biomarker, it should be associated with the intensity of the key pathological features of Alzheimer’s disease — plaques and tangles, as demonstrated for blood p-tau.14,15,19,66 Plasma BD-tau correlated with neuritic and diffuse amyloid-plaque and NFT counts, and additionally with CSF and plasma Aβ42 and p-tau. Moreover, plasma BD-tau correlated with NfL only in Alzheimer’s disease but not in other neurodegenerative diseases, further supporting its Alzheimer’s disease specificity. Together, the results demonstrate that plasma/serum BD-tau is an Alzheimer’s disease-type neurodegeneration biomarker that associates with principal pathological features of the disease. Future studies will aim to elucidate what neurodegenerative process(es) in Alzheimer’s disease that plasma BD-tau reflects — for e.g. neuronal injury intensity (such as CSF t-tau), loss and shrinkage of the neuropil (such as by using structural MRI) or functional neuronal impairment including glucose hypometabolism (such as by using FDG PET).
Despite serum BD-tau levels being significantly lower in non-Alzheimer’s disease versus Alzheimer’s disease, the concentrations in individuals with frontotemporal lobal degeneration, particularly those carrying GRN mutations, tended to be further lower than those of control participants and the other non-Alzheimer’s disease groups (including progressive supranuclear palsy and corticobasal syndrome; Fig. 5). While this observation might be unexpected, highly comparable results have been reported for serum p-tau181 in the same population.56 Together, the findings may suggest a remarkable decrease in the secretion of CNS-specific biomarkers into the bloodstream in frontotemporal lobal degeneration. Conversely, the high increases in serum NfL for the GRN mutation carriers is corroborated in previous independent studies.58 These results deserve further investigation in other cohorts.
What is the value of plasma BD-tau since plasma p-tau also differentiates Alzheimer’s disease from other neurodegenerative diseases? First, we need separate biomarkers of amyloid, tau and neurodegeneration as stipulated in the AT(N) and the International Working Group frameworks.1–3 Biomarkers of neurodegeneration are not interchangeable with those that reflect amyloidosis or tau phosphorylation. The frameworks also allow for flexibility to include novel biomarkers including those identified in other biofluids. More recently, plasma Aβ42/Aβ40 and p-tau biomarkers have shown great potential to substitute for CSF A and T biomarkers in the AT(N) scheme.67 However, unlike in CSF where t-tau shows specificity to Alzheimer’s disease, there is currently no blood biomarker that reflects neurodegeneration specific to Alzheimer’s disease. NfL in blood does not meet this requirement because it reflects neurodegeneration shared among multiple neurodegenerative diseases.21,64 Plasma BD-tau shows high potential as a neurodegeneration biomarker of the Alzheimer’s type. We anticipate that combining plasma BD-tau with p-tau and possibly Aβ42/Aβ40 will increase accuracy of a blood biomarker-based diagnosis of Alzheimer’s disease by increasing its agreement with results obtained at autopsy or by using CSF or neuroimaging biomarkers.
In the present study, we report a novel blood biomarker specific for BD-tau. Validation showed strong correlations between paired blood and CSF measures, and we verified a high performance to specifically identify Alzheimer’s disease-type neurodegeneration. Future studies will further address the characteristics of this novel biomarker, including exploring its longitudinal changes across the Alzheimer’s disease continuum in both sporadic and familial Alzheimer’s disease, associations with neuroimaging AT(N) biomarkers and the influence of genetic risks (e.g. APOE ε4). Additionally, we will verify the generalizability of the biomarker in diverse, multi-ethnic cohorts from a variety of populations. Furthermore, we will characterize BD-tau in disorders that CSF t-tau is known to be increased in, including acute traumatic brain injury and Creutzfeldt–Jakob disease.
References & additional information
See the original publication
About the authors & affiliations
Fernando Gonzalez-Ortiz,1 Michael Turton,2 Przemysław R. Kac,1 Denis Smirnov,3 Enrico Premi,4 Roberta Ghidoni,5 Luisa Benussi,5 Valentina Cantoni,4 Claudia Saraceno,5 Jasmine Rivolta,4 Nicholas J. Ashton,1,6,7,8 Barbara Borroni,4 Douglas Galasko,3 Peter Harrison,2 Henrik Zetterberg,1,9,10,11,12 Kaj Blennow1,9 and Thomas K. Karikari1,13
1 Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy,
University of Gothenburg, Gothenburg 405 30, Sweden
2 Bioventix Plc, Romans Business Park, Farnham, Surrey GU9 7SX, UK
3 University of California, San Diego and Shiely-Marcos Alzheimer’s Disease Research Center, La Jolla, CA 92037, USA
4 Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, BS 25121, Italy
5 Molecular Markers Laboratory,
IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia 25121, Italy
6 Wallenberg Centre for Molecular and Translational Medicine,
University of Gothenburg, Gothenburg 405 30, Sweden
7 King’s College London, Institute of Psychiatry, Psychology & Neuroscience, Maurice Wohl Clinical Neuroscience Institute, London, SE5 8AF, UK
8 NIHR Biomedical Research Centre for Mental Health & Biomedical Research Unit for Dementia at South London & Maudsley NHS Foundation, London, SE5 8AF, UK
9 Clinical Neurochemistry Laboratory,
Sahlgrenska University Hospital, Mölndal, 431 80, Sweden
10 Department of Neurodegenerative Disease,
UCL Institute of Neurology, London, WC1N 3BG, UK
11 UK Dementia Research Institute at
UCL, London, WC1E 6BT, UK
12 Hong Kong Center for Neurodegenerative Diseases, Shatin, N.T., Hong Kong, China
13 Department of Psychiatry,
University of Pittsburgh, Pittsburgh, PA 15213, USA
Acknowledgements
The authors thank all participants of the research cohorts studied here and their caregivers for their invaluable time, support and biospecimen donations. We also thank research and medical staff at the University of Gothenburg, the Sahlgrenska University Hospital, the UCSD Shiley-Marcos ADRC, San Diego, and the University of Brescia for their collaborative support. Furthermore, we are grateful to Celia Hök Fröhlander for her immense support with biofluid sample processing and aliquoting.












