This is a republication of the white paper below, with the title above, preceded by an Executive Summary by the author of the blog.
Improving Health Care Value — The Case for Disease Registries
Combining Improved Outcomes with Lower Costs
Stefan Larsson and Peter Lawyer
08 DECEMBER 2011
Key messages
Summarized by Joaquim Cardoso MSc.
April 27, 2022
What is the problem?
- Despite decades of utilization reviews and cost controls, health care costs continue to rise in many developed countries-often to unsustainable levels.
What is one of the solutions?
- One approach has already shown great potential to improve the quality of care: patient registries that track outcomes in a population of patients with the same diagnosis (for example, acute myocardial infarction) or in a population of patients who have undergone a common medical procedure (for instance, cataract surgery).
- … a registry is as “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease [or] condition . . . .”
- This definition is accurate as far as it goes, but we take a broader view. We see disease registries not only as systems for the collection and analysis of data on health outcomes but also as important institutional catalysts for efforts to improve those outcomes over time.
Who is the benchmark in disease registries?
- Sweden has been an international pacesetter in the establishment of disease registries, with some dating back to the 1970s.
- Today, Sweden boasts nearly 90 registries that cover more than 25 percent of total national health expenditures.
- … Sweden has the best health-care outcomes in Europe, even though its health-care costs, as a percentage of GDP, hover around the European average of roughly 9 percent.
- By contrast, the U.S., which has the highest per capita costs, spends 17.6 percent of GDP on health care
How does it work?
- By identifying variations in outcomes within the same population, registries make it possible to benchmark and assess comparative performance at the clinic, regional, national, or even international level.
- Systematic quality improvement of this type, we believe, can also have the virtuous side effect of lowering total health-care costs for a given condition.
- What’s more, by putting the responsibility for improved quality squarely in the hands of physicians and other health-care practitioners, registries organize and engage the medical community around the common goal of better health-care value.
What is the business case?
- by investing $70 million annually in disease registries, data analysis resources, and IT infrastructure, Sweden could reduce its annual growth in health care spending from an estimated 4.7 percent to 4.1 percent [a 13% cost reduction in GDP terms)]
- The estimated cumulative return totaled more than $7 billion in reduced direct health-care costs over ten years.
Extending the Reach of Disease Registries
- … disease registries can function as powerful platforms for improving health outcomes, lowering health care costs, and thus improving health care value.
What is the Evidence of Improved Health Outcomes?
Sweden’s Acute Coronary-Care Registry.
- Between 1998 and 2009, Sweden decreased the average 30-day mortality rate for patients who experience an acute heart attack by 65 percent and the one-year mortality rate by 49 percent.
Sweden’s Hip-Replacement Registry.
- Between 1979 and 1989, Sweden reduced its ten-year postsurgery revision frequency for cemented fixations … threefold, from 9 percent of patients operated on in 1979-still among the lowest of the countries reporting-to 3 percent of those operated on in 1989.
Sweden’s Cataract Registry.
- … the incidence of postoperative endophthalmitis in Sweden declined from 0.11 percent of all cataract-surgery cases in 1998 to 0.02 percent in 2009, by far the lowest we have observed in published data [ 82% reduction in the number of cases]
U.S. Cystic Fibrosis Registry
- … the decline in the Pseudomonas infection rate accelerated significantly, so that by 2009 it had reached 51.7 percent [a 14% reduction from 1995 to 2009–14 years]
Hip Surgery
- Although Australian hip surgeons still use them as a low-cost option for the very elderly (those over 85 years of age), overall usage has been cut in half-from 68 percent of cases in 2000 to 34 percent in 2009 [a 50% reduction in the number of cases]
Selected Cost reduction potentials for the US
Hip Arthroplasty Revision
- … if the U.S. health-care system improved its revision rate by 2015 to the level of Sweden’s today, it would avoid $2 billion of the expected $24 billion in total costs [around 9% reduction in costs]
Postoperative Endophthalmitis
- If the U.S. had achieved similar incidence levels, it could have prevented about 8,500 of these cases [a 75% reduction in the number of cases]
Pseudomonas Infection in Cystic Fibrosis
- these improvements — in treating cystic fibrosis — represent $23 million per year in avoided infection-related costs, or $230 million during the entire ten-year period, which equates to roughly 2 percent of the total costs of cystic fibrosis care [a 98% reduction in the number of cases]
Conclusion
- After decades of struggle to control rising health-care costs, it is clear that the conventional methods have had only limited effectiveness.
- There is, however, a promising alternative: improving patient outcomes and focusing on health care value.
- Sweden, which provides high-quality care at relatively low cost, offers a living laboratory of what can be accomplished when a strong system of disease registries provides clear goals for caregivers.
- Likewise, disease registries in the U.S., Denmark, Australia, the U.K., and other countries have witnessed similar improvements over time by engaging doctors and other clinical professionals to identify, compare, and adopt best practices that transform care-and lower total costs.
ORIGINAL PUBLICATION (full version)

Improving Health Care Value — The Case for Disease Registries
Introduction
Despite decades of utilization reviews and cost controls, health care costs continue to rise in many developed countries-often to unsustainable levels.
Not only have traditional approaches failed to contain costs to a significant degree, they have often come at the price of demoralizing the medical community.
Physicians and other health-care professionals have protested, and at times resisted, what they see as the imposition of inappropriate cost metrics on patient care.
Despite decades of utilization reviews and cost controls, health care costs continue to rise in many developed countries-often to unsustainable levels.
In response to the failure of conventional cost controls to rein in health care expenditures, leading policymakers have recommended that health care reform efforts emphasize improving value by weighing well-defined patient outcomes against treatment costs.
According to the Institute of Medicine, the health arm of the U.S. National Academy of Sciences, “A variety of strategies are beginning to be employed throughout the health system to address the central issue of value, with the goal of improving the net ratio of benefits obtained per dollar spent on health care.”
One approach has already shown great potential to improve the quality of care: patient registries that track outcomes in a population of patients with the same diagnosis (for example, acute myocardial infarction) or in a population of patients who have undergone a common medical procedure (for instance, cataract surgery).
For reasons of simplicity (and to distinguish these registries from other types, such as drug exposure or device registries), we refer to them as “disease registries.”
One approach has already shown great potential to improve the quality of care:
patient registries that track outcomes in a population of patients with the same diagnosis (for example, acute myocardial infarction) or in a population of patients who have undergone a common medical procedure (for instance, cataract surgery).
Most discussions of disease registries portray them primarily as repositories of data useful for outcomes research.
A handbook published by the U.S. Agency for Healthcare Research and Quality defines a registry as “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease [or] condition . . . .”
… a registry as “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease [or] condition . . . .”
This definition is accurate as far as it goes, but we take a broader view.
This definition is accurate as far as it goes, but we take a broader view.
We see disease registries not only as systems for the collection and analysis of data on health outcomes but also as important institutional catalysts for efforts to improve those outcomes over time.
We see disease registries not only as systems for the collection and analysis of data on health outcomes but also as important institutional catalysts for efforts to improve those outcomes over time.
By identifying variations in outcomes within the same population, registries make it possible to benchmark and assess comparative performance at the clinic, regional, national, or even international level.
In-depth analysis of the causes behind variations in performance can lead to the identification of best practices.
Active dissemination of those best practices and support to enable their adoption improve outcomes and reduce variations in clinical practice.
By identifying variations in outcomes within the same population, registries make it possible to benchmark and assess comparative performance at the clinic, regional, national, or even international level.
Systematic quality improvement of this type, we believe, can also have the virtuous side effect of lowering total health-care costs for a given condition.
What’s more, by putting the responsibility for improved quality squarely in the hands of physicians and other health-care practitioners, registries organize and engage the medical community around the common goal of better health-care value.
Systematic quality improvement of this type, we believe, can also have the virtuous side effect of lowering total health-care costs for a given condition.
What’s more, by putting the responsibility for improved quality squarely in the hands of physicians and other health-care practitioners, registries organize and engage the medical community around the common goal of better health-care value.

Disease Registries and Value-Based Health Care
Since 2009, The Boston Consulting Group has been studying disease registries as a potential model for value-based health care, an approach to controlling health care costs that emphasizes maximizing value, defined as patient outcomes divided by costs.
This research began in 2009, when a group of senior health-care leaders in Sweden asked BCG to analyze that country’s disease registries and evaluate the opportunities and costs involved in expanding the registry model to include other conditions.
Sweden has been an international pacesetter in the establishment of disease registries, with some dating back to the 1970s.
Today, Sweden boasts nearly 90 registries that cover more than 25 percent of total national health expenditures.
About a third collect patient data on more than 90 percent of all Swedish patients diagnosed with a given condition or undergoing a common procedure, and many have been in place long enough to provide unique longitudinal data.
Today, Sweden boasts nearly 90 registries that cover more than 25 percent of total national health expenditures.
A recent study also found Sweden to have the best health-care outcomes in Europe, even though its health-care costs, as a percentage of GDP, hover around the European average of roughly 9 percent.
By contrast, the U.S., which has the highest per capita costs, spends 17.6 percent of GDP on health care.
… Sweden has the best health-care outcomes in Europe, even though its health-care costs, as a percentage of GDP, hover around the European average of roughly 9 percent.
Our study concluded that by investing $70 million annually in disease registries, data analysis resources, and IT infrastructure, Sweden could reduce its annual growth in health care spending from an estimated 4.7 percent to 4.1 percent.
The estimated cumulative return totaled more than $7 billion in reduced direct health-care costs over ten years. Partly as a result of our study, in September 2011, the Swedish government declared the expansion of Sweden’s network of registries a national priority, and it is now committed to increasing its direct financial support nearly fivefold-from $10 million to $45 million per year-by 2013.
… by investing $70 million annually in disease registries, data analysis resources, and IT infrastructure, Sweden could reduce its annual growth in health care spending from an estimated 4.7 percent to 4.1 percent.
The estimated cumulative return totaled more than $7 billion in reduced direct health-care costs over ten years.
Encouraged by the initial results of the Swedish study, BCG expanded its research in 2010 to analyze the impact of disease registries in other countries.
Our study had four primary goals:
- to document outcomes improvement in patient populations covered by disease registries;
- to identify what role, if any, the registries played in achieving these results;
- to quantify (where data were available) the cost savings made possible by those improvements in the form of avoided health-care costs;
- and to estimate the potential impact of similar improvements in the U.S. Through these efforts, we hoped to establish a compelling “proof of concept” that registries are a powerful mechanism for improving health care value.

We studied 13 registries in five countries and across six major disease areas. Although many of the registries we studied were in Sweden, the majority were located in other countries, including Australia, Denmark, the U.K., and the U.S. (See “Participating Disease Registries,” below.)
In this report, we focus on registries covering four major disease areas or medical procedures: acute myocardial infarction, cataract surgery, cystic fibrosis, and hip arthroplasty.

In addition to analyzing both published and unpublished registry data, we also interviewed approximately 40 health-care professionals-registry experts, physicians who use registry data, and health care policymakers-to gain insight into how the registries function and to identify the mechanisms by which they are able to influence clinical practice.

Evidence of Improved Health Outcomes
Our study identified many instances where registries have contributed to significant improvements in health outcomes considered indicative of high-quality care by the relevant medical specialists.
Consider some illustrative examples.
Sweden’s Acute Coronary-Care Registry.
Founded in 1991, the Register of Information and Knowledge about Swedish Heart Intensive-care Admissions (since 2009, a part of Swedeheart, Sweden’s national registry for acute coronary care) collects data from all 74 of the nation’s major medical centers and covers approximately 80 percent of patients who suffer from acute myocardial infarction (heart attack).
The registry tracks well-accepted clinical outcomes standards, such as 30-day and one-year mortality rates, as well as proven input and process metrics, such as hospital compliance with nine interventions recommended by the European Society of Cardiology.
In addition to collecting and sharing data among Sweden’s hospitals and cardiologic specialists, the registry actively promotes adherence to clinical guidelines.
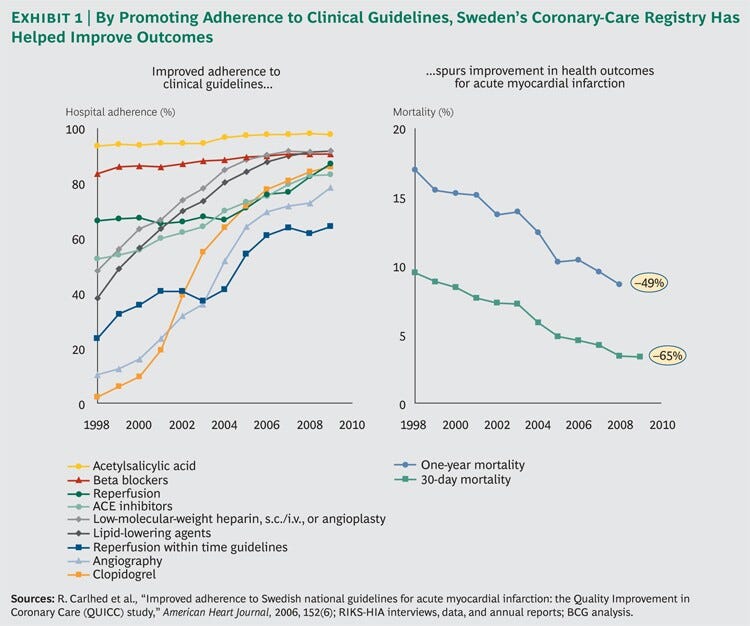
Between 1998 and 2009, Sweden decreased the average 30-day mortality rate for patients who experience an acute heart attack by 65 percent and the one-year mortality rate by 49 percent. (See Exhibit 1.)
Between 1998 and 2009, Sweden decreased the average 30-day mortality rate for patients who experience an acute heart attack by 65 percent and the one-year mortality rate by 49 percent.
Sweden’s Hip-Replacement Registry.
Total-hip arthroplasty, the replacement of a hip joint with an artificial prosthesis, is a common operation.
In the U.S., more than 200,000 primary total-hip replacements are done each year for people over 80 years of age, and the number is expected to reach 600,000 annually by 2030.
Although an effective procedure, total-hip arthroplasty fails for some patients, whether due to postoperative infection or normal wear and tear of the prosthetic hip, and the failure rate increases over time after surgery.
In such cases, a second procedure, known as a “revision,” is required to repair or replace the implant.
Founded in 1979, the Swedish Hip Arthroplasty Register is the world’s oldest hip-replacement registry and, with more than 24,000 registrations each year, currently has some 400,000 procedures in its database.
Between 1979 and 1989, Sweden reduced its ten-year postsurgery revision frequency for cemented fixations (the most common form of hip replacement) threefold, from 9 percent of patients operated on in 1979-still among the lowest of the countries reporting-to 3 percent of those operated on in 1989.
This represents a dramatic improvement given that there were no major changes in surgical indications during that period.
Between 1979 and 1989, Sweden reduced its ten-year postsurgery revision frequency for cemented fixations … threefold, from 9 percent of patients operated on in 1979-still among the lowest of the countries reporting-to 3 percent of those operated on in 1989.
Sweden’s Cataract Registry.
Established in 1992, Sweden’s National Cataract Register (NCR) systematically collects outcomes data and mobilizes active support for quality improvement.
Currently, all public eye clinics and ophthalmology departments in Sweden and 98 percent of all surgeries performed by private clinics are covered by the registry’s database, which includes more than 1 million records representing 95.6 percent of all cataract extractions performed in Sweden since 1992.
Owned by the Swedish Ophthalmological Society, the registry has been an active participant in the development of national guidelines that have improved cataract surgery outcomes in Sweden.
Consider the example of postoperative endophthalmitis, a rare but severe infection of both the anterior and posterior segments of the eye that is among the complications that can occur during cataract surgery.
In countries with developed cataract surgery, there are only one or two cases per 1,000 operations. But when postoperative endophthalmitis does occur, its effects can be severe. Between 30 and 50 percent of patients who develop the infection become extremely disabled and even legally blind.
Since postoperative endophthalmitis is so rare, few hospitals or medical centers can develop a large enough reference sample to produce reliable results.
In 1997, Sweden’s cataract registry began collecting nationwide data on the condition. Over the next decade, the registry identified a series of specific risk factors associated with the disease, as well as best practices that improved outcomes.
In 1998, for example, NCR recommended the use of the antibiotic cefuroxime, and by 2001 had demonstrated the drug’s effectiveness as a prophylactic treatment (the administration of cefuroxime during cataract surgery has now become a European standard).
In 2001, the registry started measuring the incidence of capsular rupture, a condition in which the capsule surrounding the lens comes into contact with vitreous (the gelatinous substance that fills the space between the front and back of the eye).
When capsular rupture occurs, infection is more likely, and by 2004, NCR had demonstrated that it, too, was a risk factor for postoperative endophthalmitis. By 2006, registry researchers had become convinced that thin or compromised lens capsules and other aspects of eye anatomy were additional risk factors.
They helped create an early-warning system in which hospitals were encouraged to send their most complicated cases to the most experienced surgeons and hospitals in the country.
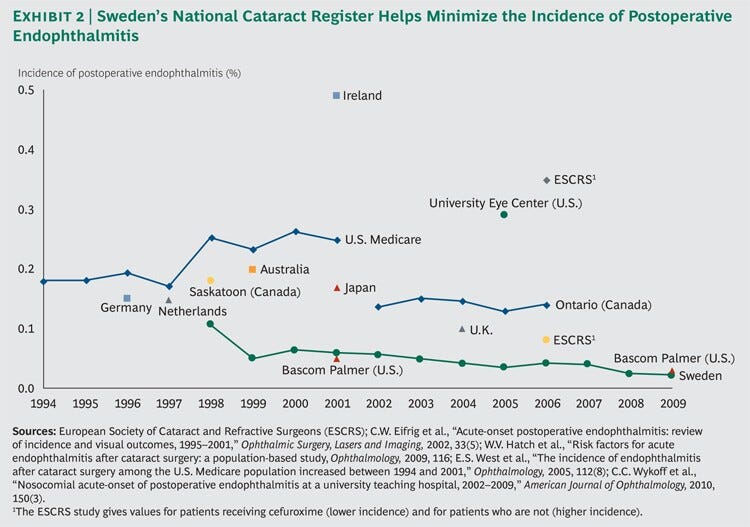
As a result of these efforts, the incidence of postoperative endophthalmitis in Sweden declined from 0.11 percent of all cataract-surgery cases in 1998 to 0.02 percent in 2009, by far the lowest we have observed in published data.
The average Swedish ophthalmology clinic boasts results on par with those of the Bascom Palmer Eye Institute in Miami, which is consistently rated the best ophthalmic hospital in the U.S. and is among the best in the world. (See Exhibit 2.)
… the incidence of postoperative endophthalmitis in Sweden declined from 0.11 percent of all cataract-surgery cases in 1998 to 0.02 percent in 2009, by far the lowest we have observed in published data [ 82% reduction in the number of cases]
U.S. Cystic Fibrosis Registry.
Established in 1966, the Cystic Fibrosis Foundation Patient Registry is one of the few managed by a patient group, the U.S. Cystic Fibrosis Foundation.
The registry collects data from all 115 certified cystic-fibrosis centers in the country and tracks more than 25,000 patients.
Among the data collected are information on growth, genotype, lung function results, pancreatic enzyme use, transplantation, and complications such as lung infections, diabetes, liver disease, and death.
A common complication of cystic fibrosis is infection by Pseudomonas, an opportunistic pathogen that is one of the most serious and difficult to treat hospital-acquired infections.
It colonizes the lungs of patients with cystic fibrosis, contributing to the chronic progressive pulmonary disease and high death rate associated with the condition.
Since 1995, the Pseudomonas rate in U.S. cystic-fibrosis patients has declined by more than 14 percent, and there are indications that efforts by the cystic fibrosis registry were an important catalyst in this improvement.
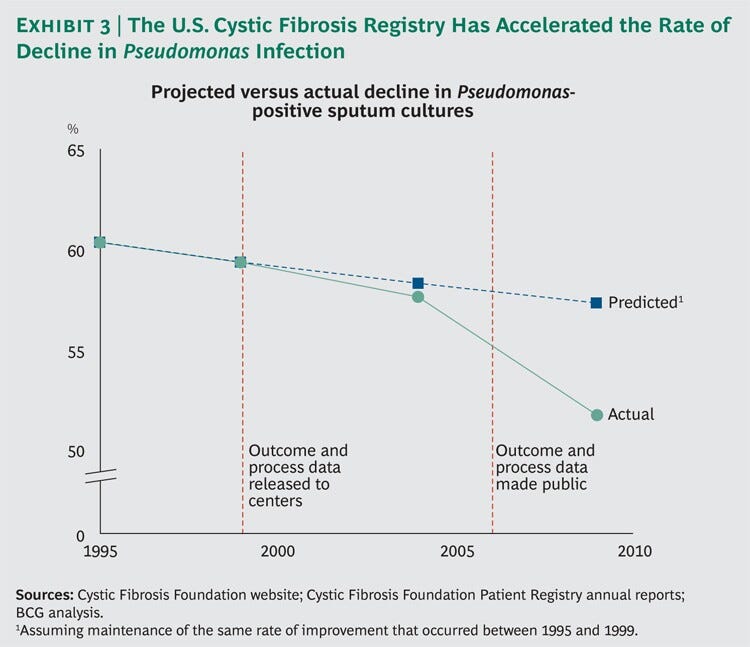
Between 1995 and 1999, the decline in Pseudomonas-positive sputum cultures was relatively modest-from 60.3 percent to 59.3 percent.
A straight extrapolation of this trend line would yield a 57.3 percent positive-culture rate by 2009.
However, in 1999, the registry began providing each center with data on its own performance compared with a national benchmark; in 2006, the data were made available to the public.
With greater visibility and, therefore, increased focus on this key indicator, the decline in the Pseudomonas infection rate accelerated significantly, so that by 2009 it had reached 51.7 percent. (See Exhibit 3.)
… the decline in the Pseudomonas infection rate accelerated significantly, so that by 2009 it had reached 51.7 percent [a 14% reduction from 1995 to 2009–14 years]
Data-Based Continuous Improvement
In the absence of a randomized controlled study, it is impossible to isolate the precise impact of registry activities from other factors on improved health outcomes.
It is likely that some portion of the improvements described above is the product of secular improvements in clinical practice that would have occurred even in the absence of a robust disease registry.
However, when one looks closely at how the disease registries we studied operate, there are many indications that they play an active-and in some cases, a leading-role in encouraging changes in clinical practice that help generate improved health outcomes.
Our research identified four characteristics, in particular, that allow registries to play this role effectively.
- Comprehensive, High-Quality Data
- A Bias Toward Data Transparency.
- Active Engagement with the Clinical Community.
- Cross-Border Collaboration.
Comprehensive, High-Quality Data.
The registries we studied pay significant attention not only to collecting comprehensive data but also to making sure the information is reliable.
In addition to the data it collects on heart attack patients, for example, Swedeheart also covers 100 percent of patients undergoing angiography, angioplasty, and cardiac surgery and nearly 100 percent of acute coronary syndrome (ACS) patients admitted to coronary-care units.
The registry collects baseline data on 106 variables for patients with ACS, with another 75 variables relating to secondary prevention collected after 12 to 14 months. The data are registered online directly by the caregiver.
In addition to automatic error-checking routines for range and consistency, a monitor visits approximately 20 randomly selected hospitals each year to compare data entered into the registry with the information in the records of 30 to 40 randomly chosen patients.
In 2007, this data-validation process demonstrated an agreement of 96.1 percent.
A similar study of 574 operations for cataract surgery at five Swedish clinics showed a similar high degree of agreement (95.4 percent), suggesting that the reliability of these registry databases is quite high. (See “Meeting the Data Challenge.”)
A Bias Toward Data Transparency.
The registries we studied do not simply collect data. As the cystic fibrosis example suggests, they also work actively to make the data transparent -to health care practitioners and then, once the data collection process and outcomes metrics have been fully vetted, to the public as well.
Broad access to registry data has a demonstrated impact on the rate of clinical improvement.
In 2005, the Swedish heart-attack registry created a quality index that tracks how well the nation’s cardiac hospitals are complying with national clinical guidelines.
At first, the registry published only aggregate data at the regional level, but in late 2006 it decided to make public both the index scores and actual patient survival rates for each of the country’s 74 hospitals.
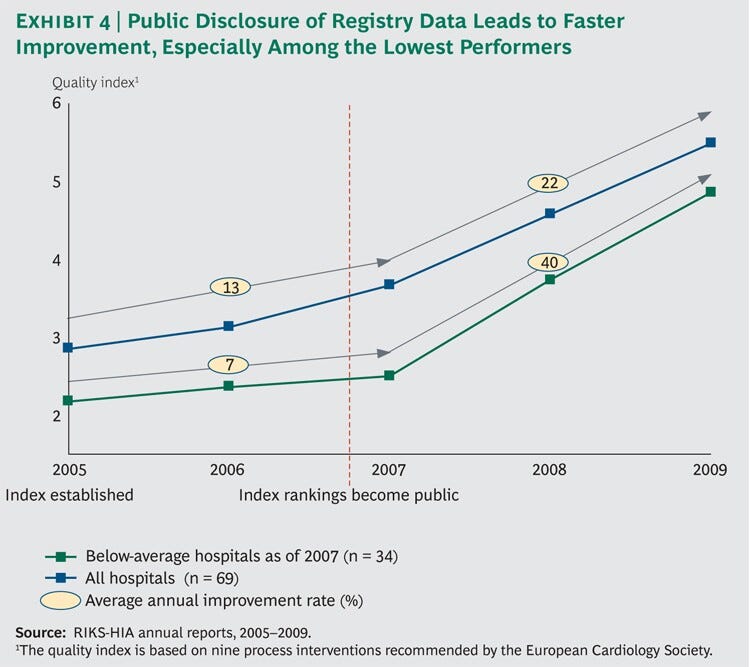
A review of the results shows a sharp inflection point after public disclosure. (See Exhibit 4.)
From 2005 through 2007, the average quality-index score improved by 13 percent per year.
Meanwhile, the bottom half of performers improved by only 7 percent, indicating a widening quality gap between above-average and below-average clinics.
From 2007 through 2009, the period after the data were made fully public, the overall rate of improvement almost doubled to 22 percent per year. But bottom-half performers improved their quality scores by 40 percent, decisively narrowing the quality gap.
Karlstad Hospital, located in southeastern Sweden, illustrates how data transparency can inspire clinical engagement, create focus on a clear goal, and in the process transform care.
In 2005, Karlstad had one of the lowest rankings on the quality index. In response, the hospital initiated a change effort to reorganize the total care cycle and improve adherence to national guidelines.
By 2007, the hospital had improved its rank to 43 out of Sweden’s 74 cardiac-care centers, but it was still subjected to negative press coverage when the rankings became public.
Over the next two years, Karlstad boosted its ranking to 22, cut its 30-day mortality rate from 9 percent to 4 percent, and improved its one-year mortality rate from 13.5 percent to 5.2 percent, substantially below the national average.
According to one cardiac-care physician at Karlstad, “The media coverage played an important role in our improvement work, but the foundation was our observations from the registry.”
Cardiac-care clinicians throughout Sweden seem to agree. The registry conducts an annual survey of the physicians and nurses who are the formal contacts for the registry at Sweden’s cardiac-care centers.
In 2010, the survey received 124 responses (a response rate of 88.5 percent). Seventy-seven percent of the responding physicians and 74 percent of the nurses either strongly agreed or partly agreed with the statement that “publicly available results have made it easier to motivate and implement change.”
And 86 percent and 78 percent, respectively, either strongly agreed or partly agreed that “the Quality Register enhances our speed of adoption of best practice guidelines.”
Active Engagement with the Clinical Community.
In effect, the registries we studied function as a valued community platform, working actively to engage physicians and other clinical staff in the shared task of improving the quality of care and serving as a clearing-house for the dissemination and sharing of best practices.
The U.S. cystic fibrosis registry, for instance, has established a benchmarking team made up of clinicians with strong reputations in the cystic fibrosis medical community and strong ties to the various centers.
The team identifies top performers, documents best practices, and encourages site visits to high-performing centers to observe patient management and identify novel diagnostic and therapeutic approaches.
The registry, like many of the others we studied, also spreads best practices nationwide through a regular program of national conferences, local meetings, newsletters, and word of mouth.
The positive impact of this type of active intervention by disease registries has been demonstrated in Sweden. In 2002, the Uppsala Clinical Research Center conducted a study of quality improvement in coronary care.
The study selected 19 hospitals as sites for a quality improvement intervention, pairing them with 19 other hospitals with similar populations and treatment profiles.
Although the latter were recipients of registry data, they received no intervention (indeed, they were not even aware of the study).
Quality improvement teams, consisting of registry experts, the research team, and hospital clinicians, worked to help the intervention hospitals optimize their use of registry data.
The registry, meanwhile, offered these hospitals rapid feedback and continuous measurement of their efforts-including real-time national comparisons, analyses of local time series, and performance-level measurements.
By the end of the study, the intervention hospitals showed significant improvement in all five of the quality parameters the researchers tracked, whereas the reference hospitals did not.

Cross-Border Collaboration.
Finally, the disease registries we studied are increasingly functioning as a mechanism for improving outcomes not only within individual countries but also across national health-care systems.
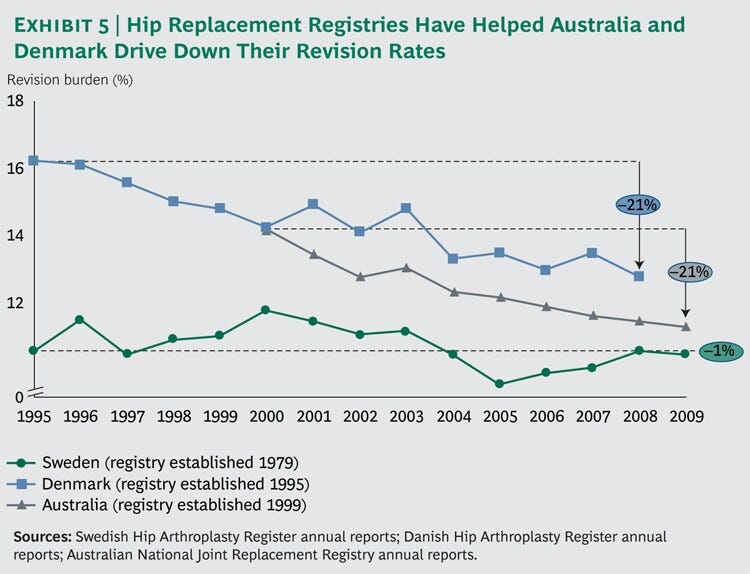
onsider the example of total-hip arthroplasty. Inspired by Sweden’s reduction of its revision burden, Denmark in 1995 and Australia in 1999 created hip replacement registries largely based on the Swedish model.
As anticipated, the revision burden of both countries dropped to near the Swedish level over the next several years. (See Exhibit 5.)
One procedure that has been especially affected by this cross-border learning is hemiarthroplasty, or partial-hip replacement.
The initial Australian data showed that monoblock implants were the most common device used for hemiarthroplasty, accounting for 68 percent of all Australian operations in 2000.
But the data also showed that monoblock implants had the highest revision rates, with 3.6 percent of patients requiring a second procedure within the first year after surgery, 5.8 percent within three years, and 6.4 percent within five years.
Meanwhile, data coming from Sweden around the same time showed that mono-block implants also carried an increased risk of dislocation.
And although they were cheaper, monoblock devices required a far more complex surgical procedure than other types of implants.
As a result of these findings, Swedish hip surgeons abandoned monoblock implants altogether.
Although Australian hip surgeons still use them as a low-cost option for the very elderly (those over 85 years of age), overall usage has been cut in half-from 68 percent of cases in 2000 to 34 percent in 2009

Combining Improved Outcomes with Lower Costs
The evidence-based continuous improvement enabled by disease registries has strong parallels to the total quality management movement that has swept through the manufacturing world in recent decades.
One of the core tenets is that boosting quality often has the beneficial side effect of lowering costs, a principle captured in the expression “quality is free.”
Despite severe data limitations, we found several instances that suggest that this phenomenon also applies to disease registries.
One of the core tenets is that boosting quality often has the beneficial side effect of lowering costs, a principle captured in the expression “quality is free.”
Hip Arthroplasty Revision.
In addition to being disruptive for patients, hip replacement revisions are expensive.
Based on registry data, we estimate that in the decade from 2000 through 2009 Sweden avoided some 7,500 revisions that would otherwise have taken place had Sweden’s revision rates been as high as those in the U.S at the same time.
At an average revision cost in Sweden of roughly $18,500 per operation, that represents approximately $140 million, or $14 million per year, in avoided costs-about 8 percent of the total cost of total-hip arthroplasty in Sweden during this period.
And since direct medical costs represent only about 20 percent of the total social costs of revision, when indirect costs are taken into account, the savings could be as much as $70 million per year.
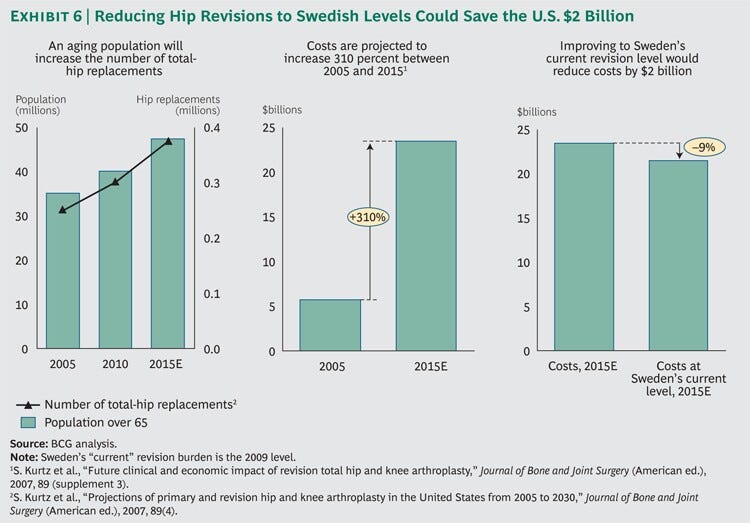
The U.S. health care system spent $6 billion on total-hip arthroplasty in 2005, and according to one estimate, these costs are expected to rise to $24 billion by 2015, an increase of 310 percent in ten years.
We estimate that if the U.S. health-care system improved its revision rate by 2015 to the level of Sweden’s today, it would avoid $2 billion of the expected $24 billion in total costs (around 9%)
Notes: 5 S. Kurtz et al., “Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030,” Journal of Bone and Joint Surgery (American ed.), 2007, 89(4), and S. Kurtz et al., “Future clinical and economic impact of revision total hip and knee arthroplasty,” Journal of Bone and Joint Surgery (American ed.), 2007, 89 (supplement 3). (See Exhibit 6.)
… if the U.S. health-care system improved its revision rate by 2015 to the level of Sweden’s today, it would avoid $2 billion of the expected $24 billion in total costs (around 9%)
Postoperative Endophthalmitis.
Most countries do not track cataract procedures-and therefore the incidence of postoperative endopthalmitis-nearly as systematically as Sweden does.
To estimate the possible savings were the U.S. to achieve Swedish incidence levels, we used a Medicare sample analyzed by researchers at Johns Hopkins that identified approximately 12,000 U.S. cases from 1998 to 2001, an incidence of approximately 0.25 percent.
By comparison, Sweden’s incidence dropped from 0.11 percent to 0.06 percent during this period.
If the U.S. had achieved similar incidence levels, it could have prevented about 8,500 of these cases [a 75% reduction in the number of cases]
Applying U.S. Medicare payments for cataract disease between 1998 and 2001 (the only time series available) and British data on the broader social costs of blindness, we estimate that preventing these 8,500 cases would have saved approximately $100 million, or about $25 million per year, in direct medical costs, and as much as $500 million, or $125 million per year, in the total medical and social costs of postoperative endophthalmitis.
Notes: 7 M. Salm et al., “Trends in cost in major eye diseases in Medicare,” 1991–2000, American Journal of Ophthalmology, 2006, 142, and J. Schmier et al., “Evaluation of Medicare costs of endophthalmitis among patients after cataract surgery,” Ophthalmology, 2007, 114(6).
If the U.S. had achieved similar incidence levels, it could have prevented about 8,500 of these cases [a 75% reduction in the number of cases]
Pseudomonas Infection in Cystic Fibrosis.
In 2008, the average cost of treating cystic fibrosis in the U.S. was approximately $43,000 per patient.
But that average disguises the heavy costs associated with Pseudomonas infection. Treatment costs for noninfected cystic-fibrosis patients averaged less than $20,000 per year, whereas treatment costs for infected patients averaged about $66,000.
Based on historical trends, we estimate that from 2000 through 2009, the cumulative improvements in infection rate cited earlier prevented approximately 5,000 infected patient years that would have occurred without the clarity and focus provided by the cystic fibrosis registry.
At an extra cost of care for infected patients of approximately $46,000 per year, these improvements represent $23 million per year in avoided infection-related costs, or $230 million during the entire ten-year period, which equates to roughly 2 percent of the total costs of cystic fibrosis care.
these improvements — in treating cystic fibrosis — represent $23 million per year in avoided infection-related costs, or $230 million during the entire ten-year period, which equates to roughly 2 percent of the total costs of cystic fibrosis care [a 98% reduction in the number of cases]

Extending the Reach of Disease Registries
Our research demonstrates that disease registries can function as powerful platforms for improving health outcomes, lowering health care costs, and thus improving health care value.
But the formation of registries in the U.S. with the scope and comprehensiveness of those found in Sweden and other nations is hampered by significant challenges.
(See “Assessing a Country’s Readiness for Value-Based Health Care” for a look at the challenges faced by various countries.)
Our research demonstrates that disease registries can function as powerful platforms for improving health outcomes, lowering health care costs, and thus improving health care value.
The U.S. health-payer system is extremely complex and fragmented, with hundreds of thousands of physicians and clinicians reporting health information via their practices, clinics, and hospitals to hundreds of public and private payers.
Reporting standards and clinical outcome metrics differ substantially across this system, even within the same specialty.
Although new government-mandated “meaningful use” requirements will encourage the widespread adoption of electronic health records, there currently exists no national mechanism to compel providers to report outcomes to disease registries.
Nor is there a unique patient identifier in place that would enable researchers to combine data across different disease states to examine the effect of complex comorbidities.
The U.S. health-payer system is extremely complex and fragmented, with hundreds of thousands of physicians and clinicians reporting health information via their practices, clinics, and hospitals to hundreds of public and private payers. …
There is an additional, cultural challenge to widespread adoption of disease registries in the U.S. In a competitive market, improving clinical outcomes can be considered a competitive advantage.
Thus, sharing results and best practices with other providers may well run counter to the interests of individual clinics and physicians.
Beyond purely competitive concerns, providers may be wary of publishing outcomes data that could lend support to medical-malpractice claims.
There is an additional, cultural challenge to widespread adoption of disease registries in the U.S. In a competitive market, improving clinical outcomes can be considered a competitive advantage.
In spite of these obstacles, however, the U.S. health-care system does have some outstanding registries, including the aforementioned
- Cystic Fibrosis Foundation Patient Registry,
- the Society for Thoracic Surgeons National Database,
- the American College of Cardiology’s CathPCI Registry,
- the U.S. Renal Data System,
- the National Trauma Data Bank, and
- the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database.
These examples show that disease registries can thrive in the U.S. health-care environment. But for more widespread and systematic usage of registries to take hold, key stakeholders would need to champion them.
In spite of these obstacles, however, the U.S. health-care system does have some outstanding registries
Physicians and Medical Professional Societies.
The disease registry model of health care outcomes improvement is founded on the principle that it is clinicians themselves who must drive change. Therefore, the national medical societies of the relevant health specialties have a leadership role to play-both in creating uniform standards for data collection and in securing the broad support and participation of practicing clinicians.
Government.
The federal government can support registries by creating a legislative and regulatory framework that facilitates their establishment and by providing seed funding to get them up and running. And with over 50 percent of health care expenditures now publicly funded, the federal government can boost registries by designing remuneration schemes to encourage clinicians to share data with registries and reward clinicians for adopting the best practices that registries identify.
Private Payers.
Private insurers will benefit from the existence of disease registries because the high-quality data they produce will help the health care system focus on genuine innovations to improve clinical outcomes and bend the health care cost curve. Therefore, they too should support the rapid expansion of registries-for instance, by investing in standardized technology platforms to make it easy to collect and share outcomes data.
Biopharmaceutical and Medical-Technology Companies.
Biopharmaceutical companies and medical-technology suppliers should recognize that they can benefit by the creation of national registries. An increasing share of new biopharmaceutical drugs are under agreements of conditional reimbursement, in which payment is partially determined by the effect on the treated population. Disease registries can be a key source of quality data for proving drug or device effectiveness, while also enabling product and service innovation to further enhance health outcomes.
Academic Biomedical Researchers.
Finally, disease registries have the potential to become an important research partner for the biomedical-research community. As clinical management increasingly broadens its focus beyond budgets and cost control, many academic medical centers will turn to registries to test scientific innovations. What’s more, the strong role predicted for genetics in the development of “personalized medicine” will need to be supported by data from retrospective observational studies that track the impact of a particular drug or procedure on the entire population of relevant patients and show its impact on health outcomes over time.
Conclusion
After decades of struggle to control rising health-care costs, it is clear that the conventional methods have had only limited effectiveness.
There is, however, a promising alternative: improving patient outcomes and focusing on health care value.
Sweden, which provides high-quality care at relatively low cost, offers a living laboratory of what can be accomplished when a strong system of disease registries provides clear goals for caregivers.
Likewise, disease registries in the U.S., Denmark, Australia, the U.K., and other countries have witnessed similar improvements over time by engaging doctors and other clinical professionals to identify, compare, and adopt best practices that transform care-and lower total costs.
After decades of struggle to control rising health-care costs, it is clear that the conventional methods have had only limited effectiveness.
There is, however, a promising alternative: improving patient outcomes and focusing on health care value.
Acknowledgments
The research described in this article was sponsored by BCG’s Health Care practice. Portions of this material appeared previously in Health Affairs.
The authors thank their Swedish registry colleagues Göran Garellick, Bertil Lindahl, and Mats Lundström for their invaluable support; their BCG colleagues Ben Berk, Elena Bloudek, Jennifer Clawson, Dan Grossman, Tarun Mahajan, Rasmus Nerman, Gabriel Osterdahl, Neil Soderlund, and Rachel Swift for their contributions to the research; and Robert Howard for assisting with the writing of this report.
Originally published at https://www.bcg.com on January 8, 2021.
Appendix: Meeting the Data Challenge
High-quality observational data is the foundation of value-based health care. Sometimes, putting that data together can be a major challenge. A well-designed data set combines the right variables with an adequate number of observations (both in terms of the number of patients and across time), all with suffi cient quality control to ensure reliable clinical reporting and consistency within and across data sets.
The recently established National Cancer Intelligence Network (NCIN) in the U.K. illustrates how clinicians and researchers are meeting the data challenge. In 2007, a consortium of cancer research groups and government offi cials came together to create a national cancer registry. Although the country had a number of regional cancer registries, they were highly fragmented and tended to focus primarily on patient mortality. The goal of the new eff ort was to create a truly nationwide registry that would track a broad variety of outcomes and enable clinicians to answer questions ranging from “How can we improve performance a er surgery?” to “How do cancer incidence and outcomes vary across regions and clinical practices?”
Cancer is a complex disease with many risk factors, some of which are still poorly understood. There have been rapid advancements in treatments and protocols, but many of these treatments are costly and their long-term impact on patient health is not yet clear, leading to high variation in the types and quality of treatment. Identifying and understanding best practices are important to improving care.
These characteristics of the disease defi ned the key data requirements for the registry. Unknown future risk factors increased the number of variables that had to be collected. It was also essential to develop longitudinal data — both across time (in order to evaluate outcomes appropriately for individuals and institutions) and across care settings (given the multiple medical specialties and institutions involved). Finally, standards had to be established both for data capture from all providers and for risk adjustment across them.
The existing regional registries captured tumor incidence and mortality data (either directly or by linking to offi cial mortality statistics), but they o en lacked detailed information on treatments and risk adjustment factors. And while common standards were available for coding, the regional registries o en diff ered slightly in what they actually captured.
The U.K.’s single-payer system and unique patient identifi ers enabled the consortium to link patient and reimbursement information for over 90 percent of all cancer suff erers in the nation. The new system harmonizes and links data across the regional registries to create a patient record with consistent data. And linking to databases outside the regional registries (for example, the cancer data set from the U.K.’s Offi ce for National Statistics, national clinical-audit data, and local-hospital episode statistics) increases the variables available for risk adjustment.
The NCIN is still working to harmonize and improve the data collected by individual registries and will eventually roll out a standard data-collection system to all regions. The new system gives researchers the ability to use aggregated patient information as a basis for secondary analysis — for example, comparing risk-adjusted mortality for individual hospitals to highlight those outside expected limits. NCIN is now able to track the risk-adjusted performance of individual hospitals in terms of 30-day mortality following colorectal cancer surgery, a key indicator of clinical eff ectiveness. The comprehensive NCIN database allows researchers to adjust for risks based on stage of tumor, age, and comorbidities and then compare performance across a large number of medical institutions to identify the best and worst performers.