The BMJ
Gareth Iacobucci
18 November 2022
The FDA has approved an immunotherapy treatment for type 1 diabetes that can delay the onset of the condition by up to three years in adults and children aged at least 8 who have stage 2 type 1 diabetes.
Tzield (teplizumab-mzwv) , delivered by intravenous injection once a day for 14 days, works by binding to certain immune system cells to delay progression to stage 3.
The treatment may deactivate the immune cells that attack insulin producing cells, while increasing the proportion of cells that help moderate the immune response.
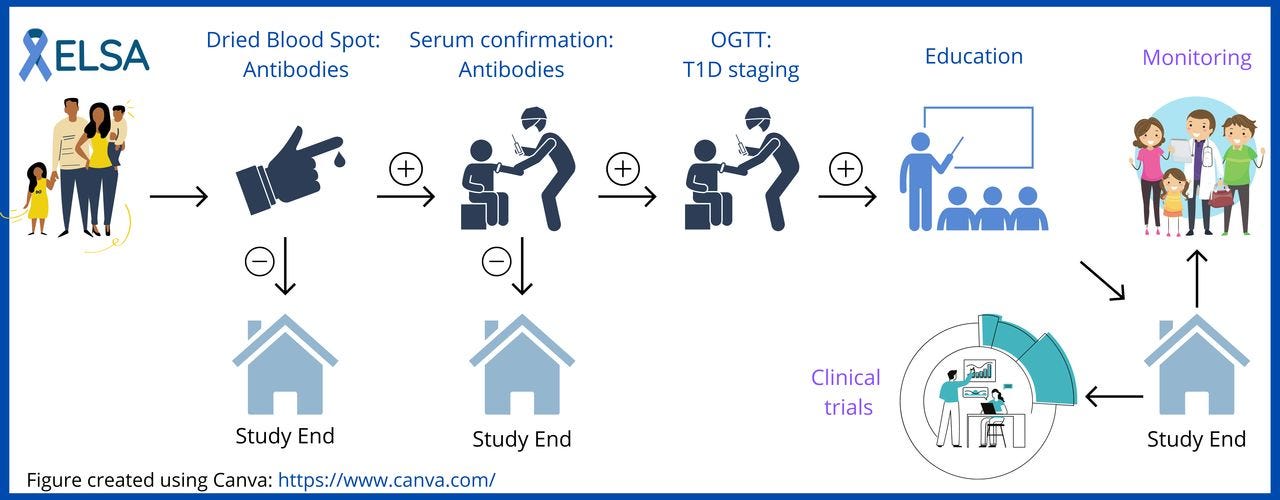
This week saw the launch of the UK based Elsa (Early Surveillance for Autoimmune Diabetes) screening trial, co-funded by Diabetes UK and the JDRF, which is seeking to identify children at high risk of type 1 diabetes.
Colin Dayan, professor of clinical diabetes and metabolism at Cardiff University School of Medicine, who leads the UK Type 1 Diabetes Immunotherapy Consortium, said the US approval of Tzield the treatment “adds weight” to the value of screening for type 1 diabetes.
“Ultimately, I hope it will lead to needing insulin to treat type 1 diabetes in childhood being a thing of the past,” he said.
Names mentioned
Colin Dayan, professor of clinical diabetes and metabolism at Cardiff University School of Medicine
Originally published at https://www.bmj.com