NEJM, Perspective
David M. Morens, M.D., Jeffery K. Taubenberger, M.D., Ph.D., and Anthony S. Fauci, M.D.
January 27, 2022
science
The past 20 years have witnessed four fatal coronavirus outbreaks: SARS (severe acute respiratory syndrome, 2002 and 2003), MERS (Middle East respiratory syndrome, since 2012), and now Covid-19 (since 2019). Scientific evidence and ecologic reality suggest that coronaviruses will emerge again in the future, potentially posing an existential threat.1 The betacoronaviruses that caused these epidemics are globally distributed in numerous species of bats. The full virologic and geographic extent of this enzootic reservoir is unknown; however, it has been increasingly spilling over into humans and other mammals.2 Because of genetic and structural receptor conservation among mammalian species, many of these animal betacoronaviruses are “preadapted” for infecting humans by binding to angiotensin-converting–enzyme 2 (ACE2) receptors, which facilitates viral spillovers and ongoing transmission.3 Some animal coronaviruses that may have pandemic potential have already been identified, and many more remain to be detected.
We need a research approach that can characterize the global “coronaviral universe” in multiple species, characterize the natural history and pathogenesis of coronaviruses in laboratory animals and in humans, and apply this information in developing broadly protective “universal” vaccines (protecting against all betacoronaviruses, and ideally all coronaviruses).
At this point, we have little understanding of the universe of endemic and potentially emerging coronaviruses.
Though coronaviruses are distributed globally, the most important betacoronavirus hot spots are in southeast Asia and contiguous areas of southern and southwestern China. Preliminary identification and sequencing of bat and other mammal-adapted coronaviruses from this region reveal rapid evolution and enormous viral complexity. Numerous bat species transmit sarbecoviruses (SARS-like viruses including SARS-CoV-2) to one another and to numerous mammals, including humans, at a high rate. Generation of new genomes through mixed infection and homologous genetic recombination leads to substantial coronaviral genetic diversity, analogous to that observed in influenza A virus evolution in wild birds, other animals, and humans. The fact that different coronaviruses, each containing most of the genome of SARS-CoV-2, have been found in one locale in Laos suggests that the building blocks for pandemic coronaviruses are being continually exchanged through genetic recombination.
To fully characterize the coronavirus ecosystem, a collaborative international effort should include extensive viral sampling of multiple bat species in multiple locales and of wild and farmed animals — including masked palm civet cats (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides), which are frequently infected with coronaviruses — as well as viral and serologic study of humans involved in wildlife and farmed animal trades and those who are occupationally exposed to bats.
Such sampling could facilitate identification of an emergence in time to prevent or control a pandemic; it would also permit study of cross-reacting epitopes, which is important for vaccine development, and support epidemiologic and serologic studies of human infection.
To gain insights into natural history and pathogenesis, it will be important to study the coronaviruses that were probably once pandemic but have now become endemic.
These four viruses ― the betacoronaviruses OC43 and HKU1 and the alphacoronaviruses 229E and NL63 ― cause mostly mild upper respiratory infections and can be studied in laboratory animals and in humans4 to characterize their epidemiology, cell tropism, elicited immune responses, cross-reactive and cross-protective epitopes, and the mechanisms by which they survive and evolve in the face of high population immunity.
Ethical human challenge studies4 can be conducted using modern genomic, transcriptomic, and immunologic tools.
Finally, we urgently need universal coronavirus vaccines.5
In the United States, the Covid-19 pandemic has been partially controlled by standard public health measures such as social distancing, masking, isolating sick and exposed people, closing places where people congregate in close quarters, and other measures, as well as by SARS-CoV-2 vaccines (two messenger RNA vaccines and one adenovirus-vectored vaccine). As important as these vaccines are, however, their protective efficacy wanes over time, necessitating booster doses. Vaccination has also been unable to prevent “breakthrough” infections, allowing subsequent transmission to other people even when the vaccine prevents severe and fatal disease.
People who have been naturally infected with SARS-CoV-2 can also be naturally reinfected, as has been shown with endemic coronaviruses, influenza viruses, respiratory syncytial virus (RSV), and many other respiratory viruses.
Moreover, immunity following natural infection with SARS-CoV-2, combined with vaccine-induced immunity, has so far not prevented the emergence and rapid spread of viral variants such as the highly transmissible delta (B.1.617.2) variant and the recently identified omicron (B.1.1.529) “variant of concern,” which as of the end of November, appeared to be highly transmissible. It remains unknown whether and how permanent protective immunity can be achieved, and whether it can prevent emergence of immune escape variants of SARS-CoV-2.
These sobering facts suggest that SARS-CoV-2 is unlikely to be eliminated, let alone eradicated; it will probably continue to circulate indefinitely in periodic outbreaks and endemics.
Meanwhile, an unknown number of animal coronaviruses, of unknown transmissibility and lethality, may well emerge in the foreseeable future. We must therefore greatly accelerate our efforts in coronavirus vaccinology.
The limitations of SARS-CoV-2 vaccines suggest that they will ultimately need to be replaced by second-generation vaccines that induce more broadly protective and more durable immunity.
We must now prioritize development of broadly protective vaccines like the universal influenza vaccines we have been working toward in recent years.
A universal coronavirus vaccine would ideally protect against SARS-CoV-2 and the many animal-derived coronaviruses that might cause future zoonotic outbreaks and pandemics.
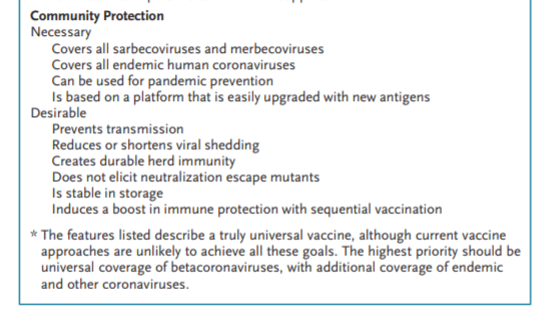
The ideal characteristics of such vaccines include properties associated with both individual and community protection in pandemics (see box).

Developing universal coronavirus vaccines will require addressing fundamental questions about the nature of coronavirus protective immunity.
In contrast to respiratory viruses that cause systemic infections (e.g., measles, rubella, varicella–zoster virus infection, and smallpox [eradicated in 1980]), nonsystemic respiratory viruses such as the endemic coronaviruses, influenza viruses, RSV, parainfluenza viruses, and SARS-CoV-2 primarily infect epithelial cells on mucosal surfaces and have limited contact with the systemic immune system. They thus elicit incomplete and transient protective immunity and allow reinfections and suboptimal responses to systemically administered vaccines.
Research will have to address several critical questions.
What are the systemic and mucosal immune correlates of protection after natural coronavirus infection and after vaccination, especially with respect to mucosal and respiratory memory B and T cells?
Which vaccine approaches will elicit immunity to multiple viral protein antigens and induce both long-term humoral and cellular memory?
What are the key humoral and cellular immune targets that will allow us to achieve robust, durable, and broadly protective immunity against the diverse and rapidly evolving betacoronaviruses? What relevant animal models of coronavirus infection and immunity can be used to adequately evaluate immune responses and vaccine efficacy?
Although clinical studies of vaccine efficacy will ultimately be needed, we must also begin now to investigate correlates of human immunity after both natural SARS-CoV-2 infection and vaccination, including by evaluating the durability of responses and their localization (mucosal and systemic).
Human challenge studies4 with the human “cold virus” coronaviruses (e.g., OC43) will probably be important. Together with studies in animals, such clinical studies could greatly improve the efficacy of universal coronavirus vaccines by helping to define immunogen design and the optimal routes and manner of vaccination.
Our ongoing experience with the current Covid-19 pandemic, together with the ever-present threat of the emergence of other potentially pandemic coronaviruses, necessitates the expeditious development of safe and broadly protective coronavirus vaccines.
This is a challenge that we must now fully commit ourselves to addressing.
This article was published on December 15, 2021, at NEJM.org.
Author Affiliations
From the National Institute of Allergy and Infectious Diseases, Bethesda, MD.
References
See the original publication. https://www.nejm.org