health transformation institute
the most comprehensive knowledge portal
for continuous health transformation
Joaquim Cardoso MSc*
Chief Researcher and Editor, and CSO
November 25, 2022
MSc* from London Business School — MIT Sloan Masters Program
Executive Summary:
What is the finding?
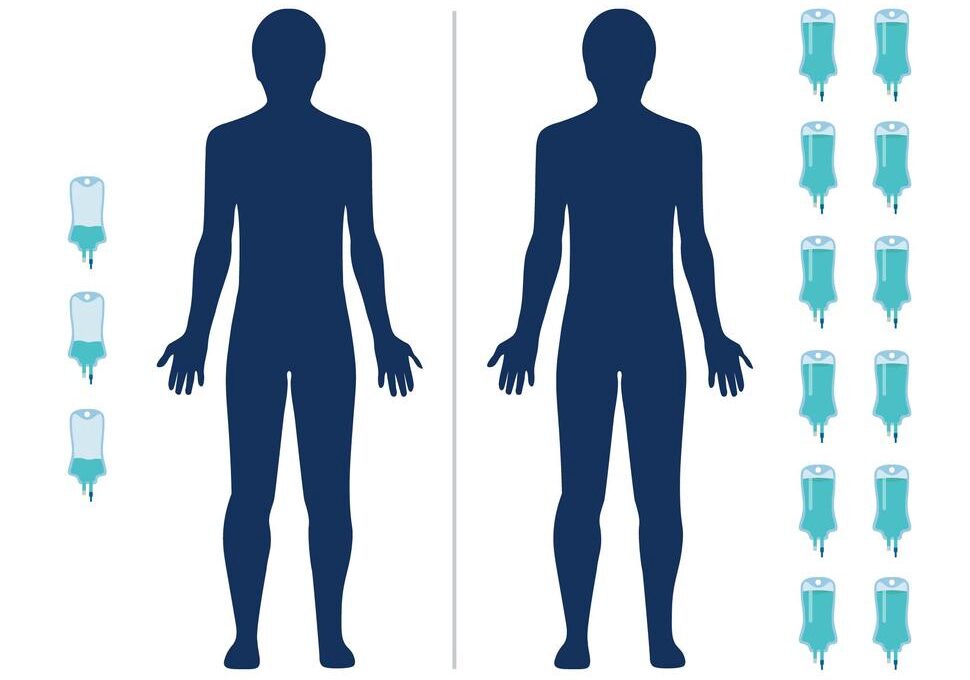
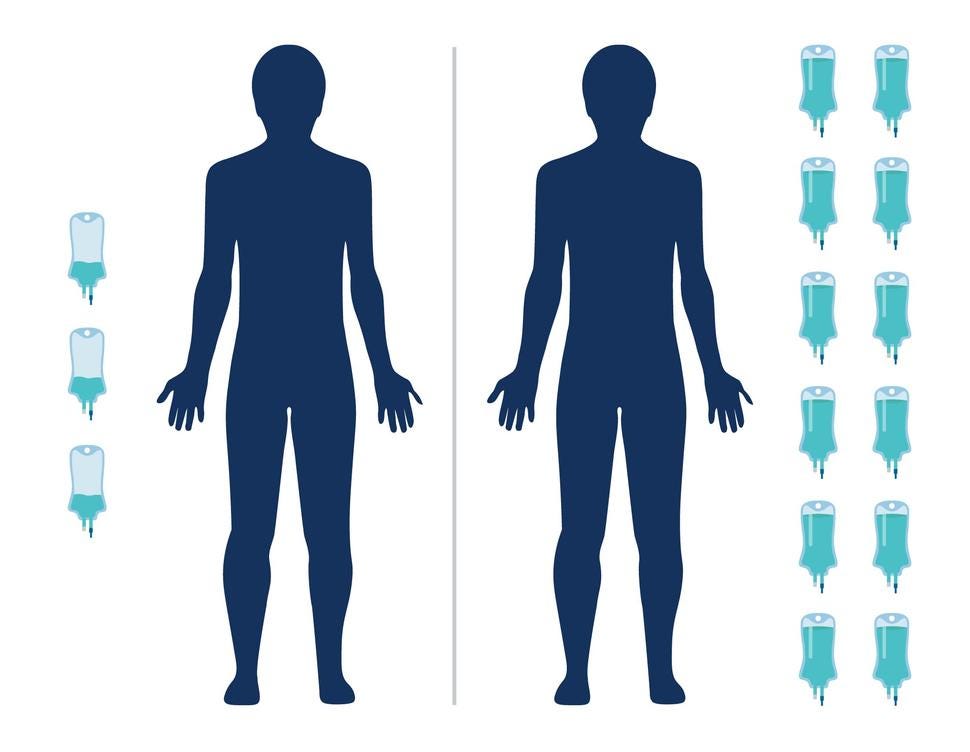
- A study in India has found that an ultra-low dose of the immunotherapy drug nivolumab (Opdivo) helped people with advanced head and neck cancer live longer
- The combination more than doubled the percentage of patients still alive 1 year later compared with the standard treatment alone
What are the benefits?
- And because the dose is 6% of what’s typically used in the United States and Europe, it is potentially more affordable
What are the implications?
- Several researchers said the results have larger implications: the potential to put the expensive immunotherapy drug within reach of many more people with cancer, particularly in low- and middle-income countries.
- Nearly 70% of the approximately 10 million cancer deaths that occur globally every year take place in low- and middle-income countries, …
What are the additional opportunities?
- The findings also highlight opportunities to test lower doses of nivolumab for people with other kinds of cancer and explore whether lower doses of other immunotherapies may also be effective
ORIGINAL PUBLICATION (national cancer institute)
Low Dose of Cancer Immunotherapy Effective in Trial in India
National Cancer Institute
Nadia Jaber
November 22, 2022
A study in India has found that an ultra-low dose of the immunotherapy drug nivolumab (Opdivo) helped people with advanced head and neck cancer live longer.
And because the dose is 6% of what’s typically used in the United States and Europe, it is potentially more affordable.
In the clinical trial, the research team added an ultra-low dose of nivolumab to the standard treatment for head and neck cancer in India.
The combination more than doubled the percentage of patients still alive 1 year later compared with the standard treatment alone, according to results published October 20 in the Journal of Clinical Oncology.
The findings may have a limited impact on head and neck cancer treatment in the United States, where the causes and treatments for these cancers are different from those in India, warned Charalampos Floudas, M.D., a head and neck medical oncologist in NCI’s Center for Cancer Research, who wasn’t involved in the work.
But several researchers said the results have larger implications: the potential to put the expensive immunotherapy drug within reach of many more people with cancer, particularly in low- and middle-income countries.
The ultra-low dose of nivolumab used in this study “decreases the cost of therapy to 5% to 9% of the cost of full-dose immunotherapy regimens,” wrote the study’s leader, Kumar Prabhash, M.D., and his colleagues.
…several researchers said the results have larger implications: the potential to put the expensive immunotherapy drug within reach of many more people with cancer, particularly in low- and middle-income countries.
The ultra-low dose of nivolumab used in this study “decreases the cost of therapy to 5% to 9% of the cost of full-dose immunotherapy regimens,” wrote the study’s leader, Kumar Prabhash, M.D., and his colleagues.
“By using a small fraction of the nivolumab dose approved in the United States and Europe, this treatment regimen dramatically reduces the financial cost of immunotherapy, with the potential to increase access and improve patient outcomes in low- and middle-income countries,” Aaron Mitchell, M.D., M.P.H., of Memorial Sloan Kettering Cancer Center, and Daniel Goldstein, M.D., of Tel Aviv University, wrote in an editorial.
Nearly 70% of the approximately 10 million cancer deaths that occur globally every year take place in low- and middle-income countries, noted Satish Gopal, M.D., M.P.H., director of NCI’s Center for Global Health, who wasn’t involved in the work.
Nearly 70% of the approximately 10 million cancer deaths that occur globally every year take place in low- and middle-income countries,
The study “is exciting and points to the value of doing cancer research and cancer clinical trials in [these] countries,” Dr. Gopal said.
The findings also highlight opportunities to test lower doses of nivolumab for people with other kinds of cancer and explore whether lower doses of other immunotherapies may also be effective, Drs. Mitchell and Goldstein noted.
The findings also highlight opportunities to test lower doses of nivolumab for people with other kinds of cancer and explore whether lower doses of other immunotherapies may also be effective

Outcomes with low-dose nivolumab
The phase 3 clinical trial included 151 people with advanced head and neck cancer that had spread ( metastasized) at the time of diagnosis or had come back after previous treatment ( recurred).
All of the participants were randomly assigned to receive standard treatment with or without low-dose nivolumab. In India, the standard treatment for advanced head and neck cancer is frequent low doses of methotrexate, erlotinib (Tarceva), and celecoxib.
Patients in the immunotherapy group were given 20 mg of nivolumab every 3 weeks via an infusion. In the United States, patients are typically given 240 mg every 2 weeks.
After one year, 43% of people in the immunotherapy group and 16% in the standard treatment group were still alive. Patients in the immunotherapy group lived for a median of 10 months, compared with 7 months for those in the standard treatment group.
“The magnitude of survival benefit that occurred with 6% of the FDA-approved dose of nivolumab for these patients is pretty remarkable,” Dr. Gopal said.
Tumors shrank or stopped growing for 59% of people in the immunotherapy group and 45% of those in the standard treatment group, the researchers found.
Among patients whose cancers shrank or stabilized, the immunotherapy treatment kept their cancer under control for longer: around 9 months versus around 3 months for the standard treatment.
Although the low dose and the full dose of nivolumab haven’t been directly compared, the survival benefit of the low dose is similar to what’s been seen in studies of the full dose, the editorialists noted.

Head and neck cancer in the United States
Around 90% of participants in the clinical trial had head and neck cancer of the mouth ( oral cavity cancer), Dr. Floudas pointed out, which is related to the use of tobacco and smokeless tobacco, including betel quid.
But in the United States, there’s a steady decline in tobacco-related oral cavity cancers and an increase in head and neck cancers that form in the back of the throat ( oropharyngeal cancer) and are related to HPV infections, he said.
Tobacco-related oral cavity tumors also tend to have more mutations than HPV-associated oropharyngeal tumors, Dr. Floudas noted, and immunotherapies often work better for tumors with a high number of mutations.
In addition, the standard treatment used in the clinical trial is not used in the United States, he added.
So, based on this one study, there’s no way to know if a low dose of nivolumab would work the same way for HPV-associated oropharyngeal cancers, Dr. Floudas said.

Saving money, time, and lives
“We’re really fortunate to have had long-standing research investments yield really important new treatments like immunotherapy,” Dr. Gopal said.
But newer treatments and tools are “often quite expensive, and how to address the escalating cost of cancer treatment-even in a high-income country like the United States-is something that we are really wrestling with,” he added.
For many people around the globe who don’t have the means to access the newest treatments, “they are generally making do with less effective options, forgoing treatment altogether, and/or financially ruining themselves trying to receive care for their cancer,” Dr. Gopal explained.
And although clinical trials often test multiple doses of a new cancer treatment, the goal is to find the highest dose that patients can tolerate because “we’re assuming that the more drug that we can give, the more effective it’s likely to be,” he noted.
But more and more, scientists and doctors are looking for ways to reduce the dose, frequency, and/or duration of cancer treatments without compromising their cancer-fighting effects.
Researchers hope that dose reductions could not just lower costs, but also lead to fewer side effects and reduced time burdens on patients and doctors, Dr. Gopal noted.
The new study also raises the possibility that lower doses of other immunotherapies could be effective for head and neck cancer as well as other kinds of cancer, Drs. Mitchell and Goldstein pointed out. The same idea could even be applied to other kinds of cancer therapy, they added.
“With lower doses and lower costs, many deaths could be prevented,” they wrote.

A global view of cancer research
The study of low-dose nivolumab is also a great example of the kinds of research questions that low- and middle-income countries are perfectly suited to tackle, Dr. Gopal said.
“Necessity is the mother of invention, [so] when you’re forced to solve a very difficult problem, it often leads to creativity, ingenuity, and innovation,” he added.
“Necessity is the mother of invention, [so] when you’re forced to solve a very difficult problem, it often leads to creativity, ingenuity, and innovation,”
“I think low- and middle-income countries may lead the way in [dose-reduction research] because in some ways, they have to do this work if they want to make these newer treatments accessible,” Dr. Gopal explained.
“Working closely with low- and middle-income country investigators and institutions, I think we are likely to gain insights that may be applicable in the United States,” he added.
Originally published at https://www.cancer.gov on November 22, 2022.
Names mentioned
Nivolumab
Charalampos Floudas, M.D., a head and neck medical oncologist in NCI’s Center for Cancer Research,
Aaron Mitchell, M.D., M.P.H., of Memorial Sloan Kettering Cancer Center, and Daniel Goldstein, M.D., of Tel Aviv University,
Satish Gopal, M.D., M.P.H., director of NCI’s Center for Global Health,
ORIGINAL PUBLICATION (journal of clinical oncology)

Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study
Vijay Maruti Patil, MBBS, MD, DM1; Vanita Noronha, MBBS, MD, DM1; Nandini Menon, MBBS, MD, DNB1; Rahul Rai, MBBS, MD1; Atanu Bhattacharjee, PhD2; Ajay Singh, MBBS, MD, DM1; Kavita Nawale, PDCR1; Shweta Jogdhankar, MSc1; Rupali Tambe, BCom1; Sachin Dhumal, BHMS1; Riddhi Sawant, PDCR1; Mitali Alone, MSc1; Devanshi Karla, MSc1; Zoya Peelay, MSc1; Shruti Pathak, MSc1; Arun Balaji, MASLP3; Suman Kumar, MBBS, DNB4; Nilendu Purandare, MBBS, DNB5; Archi Agarwal, MBBS, DNB5; Ameya Puranik, MBBS, DNB5; Abhishek Mahajan, MBBS, DNB4; Amit Janu, MBBS, DNB4; Gunjesh Kumar Singh, MBBS, MD, DM1; Neha Mittal, MBBS, MD, ; Subhash Yadav, MBBS, MD, ; Shripad Banavali, MBBS, MD1; and Kumar Prabhash, MBBS, MD, DM1
ABSTRACT
PURPOSE
The regimens approved for the treatment of advanced head and neck squamous cell carcinoma are accessible to only 1%-3% of patients in low- and middle-income countries because of their cost. In our previous study, metronomic chemotherapy improved survival in this setting. Retrospective data suggest that a low dose of nivolumab may be efficacious. Hence, we aimed to assess whether the addition of low-dose nivolumab to triple metronomic chemotherapy (TMC) improved overall survival (OS).
METHODS
- This was a randomized phase III superiority study.
- Adult patients with recurrent or newly diagnosed advanced head and neck squamous cell carcinoma being treated with palliative intent with an Eastern Cooperative Oncology Group performance status of 0–1 were eligible.
- Patients were randomly assigned 1:1 to TMC consisting of oral methotrexate 9 mg/m2 once a week, celecoxib 200 mg twice daily, and erlotinib 150 mg once daily, or TMC with intravenous nivolumab (TMC-I) 20 mg flat dose once every 3 weeks.
- The primary end point was 1-year OS.
RESULTS
- One hundred fifty-one patients were randomly assigned, 75 in TMC and 76 in the TMC-I arm.
- The addition of low-dose nivolumab led to an improvement in the 1-year OS from 16.3% (95% CI, 8.0 to 27.4) to 43.4% (95% CI, 30.8 to 55.3; hazard ratio, 0.545; 95% CI, 0.362 to 0.820; P = .0036).
- The median OS in TMC and TMC-I arms was 6.7 months (95% CI, 5.8 to 8.1) and 10.1 months (95% CI, 7.4 to 12.6), respectively (P = .0052).
- The rate of grade 3 and above adverse events was 50% and 46.1% in TMC and TMC-I arms, respectively (P = .744).
CONCLUSION
- To our knowledge, this is the first-ever randomized study to demonstrate that the addition of low-dose nivolumab to metronomic chemotherapy improved OS and is an alternative standard of care for those who cannot access full-dose checkpoint inhibitors.
About the authors & affiliations:
Vijay Maruti Patil, MBBS, MD, DM1; Vanita Noronha, MBBS, MD, DM1; Nandini Menon, MBBS, MD, DNB1; Rahul Rai, MBBS, MD1; Atanu Bhattacharjee, PhD2; Ajay Singh, MBBS, MD, DM1; Kavita Nawale, PDCR1; Shweta Jogdhankar, MSc1; Rupali Tambe, BCom1; Sachin Dhumal, BHMS1; Riddhi Sawant, PDCR1; Mitali Alone, MSc1; Devanshi Karla, MSc1; Zoya Peelay, MSc1; Shruti Pathak, MSc1; Arun Balaji, MASLP3; Suman Kumar, MBBS, DNB4; Nilendu Purandare, MBBS, DNB5; Archi Agarwal, MBBS, DNB5; Ameya Puranik, MBBS, DNB5; Abhishek Mahajan, MBBS, DNB4; Amit Janu, MBBS, DNB4; Gunjesh Kumar Singh, MBBS, MD, DM1; Neha Mittal, MBBS, MD, ; Subhash Yadav, MBBS, MD, ; Shripad Banavali, MBBS, MD1; and Kumar Prabhash, MBBS, MD, DM11Department of Medical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, India
2Section of Biostatistics, Center for Cancer Epidemiology, Tata Memorial Center, Homi Bhabha National Institute (HBNI), Mumbai, India
3Department of Speech and Therapy, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, India
4Department of Radiology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, India
5Department of Nuclear Medicine, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, India
6Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai, India
EDITORIAL: Journal of Clinical Oncology

Cost Savings and Increased Access With Ultra-Low-Dose Immunotherapy
Journal of Clinical Oncology
Aaron P. Mitchell , MD, MPH 1; and Daniel A. Goldstein , MD2,3,4,5
Key takeaways:
- In the article that accompanies this editorial, Patil et al1 reported results from a randomized clinical trial that demonstrates a significant and clinically meaningful benefit from incorporating ultra-low-dose nivolumab into the treatment of patients with advanced head and neck cancer.
- By using a small fraction of the nivolumab dose approved in the United States and Europe, this treatment regimen dramatically reduces the financial cost of immunotherapy, with the potential to increase access and improve patient outcomes in low- and middle-income countries.
Long Version
In the article that accompanies this editorial, Patil et al1 reported findings from a randomized clinical trial of nivolumab for advanced head and neck cancer.
This trial found a substantial overall survival benefit from the addition of nivolumab to a regimen combining cytotoxic (methotrexate) and targeted (erlotinib) therapies.
These findings are most notable not for the magnitude of clinical benefit but for the dose of nivolumab used to achieve them; at a flat dose of 20 mg once every 3 weeks, this represents a small fraction of the doses approved by regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency.
This trial was conducted in India, and the investigators present this alternative nivolumab dosing schedule as a viable method to substantially reduce drug costs and hence increase access to immunotherapy agents in resource-limited settings.
The nivolumab dose in this trial is approximately 6% of the FDA-approved flat dose of 240 mg once every 2 weeks.
The investigators were able to reduce costs through vial sharing, administering two full 20-mg treatments from each 40-mg vial of nivolumab.
The resulting treatment costs were < 10% the cost of nivolumab (or pembrolizumab) monotherapy at FDA-approved doses.
This cost reduction brought treatment costs within the range that is covered by the Indian national public health insurance fund, thereby enabling patients to receive treatment with an agent which would otherwise be inaccessible because of its excessively high price.
The study, therefore, represents a direct and substantial step forward within the Indian health care system and also potentially for patients with head and neck cancer treated within other health care systems that face similar budgetary constraints.
With all patients in both study arms receiving oral methotrexate 9 mg/m2 once per week, celecoxib 200 mg twice daily, and erlotinib 150 mg once daily, the addition of nivolumab 20 mg once every 3 weeks prolonged median overall survival from 6.7 months (95% CI, 5.8 to 8.1) to 10.1 months (95% CI, 7.4 to 12.6) and increased survival at 1 year from 16.3% (95% CI, 8.0 to 27.4) to 43.4% (95% CI, 30.8 to 55.3).
This is comparable with the benefit previously seen with single-agent immunotherapy for head and neck cancers, although differences in clinical setting limit direct comparison. In a platinum-refractory population, the CheckMate-141 trial found that nivolumab (at a dose of 3 mg/kg once every 2 weeks) improved median OS from 5.1 months to 7.5 months, and 1-year survival from 16.6% to 36.0%, compared with investigator’s choice of methotrexate, docetaxel, or cetuximab.2 In addition, in a platinum-refractory population, pembrolizumab improved median OS from 6.9 months to 8.4 months in the Keynote-040 trial.3
The fact that much lower doses of nivolumab produce clinical benefit comparable with conventional doses should come as no surprise, given what has long been known regarding the underlying pharmacokinetics of this drug.4
Early phase I data found similar receptor occupancy and response rates with doses ranging from 0.1 mg/kg to 10 mg/kg once every 2 weeks.5–7
For a 70 kg patient, 0.3 mg/kg per administration would work out to approximately 20 mg per administration, the flat dose used in the current study.
While higher doses persist in the plasma for a greater duration of time,5 a 3-week dosing interval appears to be sufficient to maintain therapeutic plasma concentrations even at the low dose of 0.3 mg/kg.8
Furthermore, there has already been evidence to suggest that these pharmacokinetics translate to similar clinical outcomes for ultra-low-dose nivolumab.
A randomized phase II study in advanced kidney cancer found no dose-response relationship for doses between 0.3 to 10 mg/kg once every 3 weeks in terms of progression-free survival or overall survival.9
Further clinical examples of the efficacy of ultra-low-dose nivolumab include a study in relapsed/refractory Hodgkin disease.
In this study, using a dose of 40 mg once every 2 weeks, an objective response rate of 70% was achieved.10
In this study, using a dose of 40 mg once every 2 weeks, an objective response rate of 70% was achieved.10
Although this study may directly improve cancer care in low- and middle-income countries, some may argue that the study should have limited direct implications in high-income countries.
For the population treated in this study — metastatic or locally advanced head and neck cancer among patients who are candidates only for palliative systemic therapy — the control arm therapy of low-dose methotrexate and erlotinib would be considered nonstandard.
Single-agent methotrexate is recommended as a potential palliative option,11 although at a substantially higher dose (40 mg/m2 intravenous once per week) than the 9 mg/m2 once per week used in this study.12
Erlotinib is not recommended either as a single agent or in combination; the alternative epidermal growth factor receptor inhibitor afatinib is a recommended, although nonpreferred, option in the post-platinum setting.11
The role for addition of low-dose nivolumab and the potential magnitude of benefit in settings where low-dose methotrexate plus epidermal growth factor receptor–tyrosine kinase inhibitor does not represent a baseline standard-of-care, therefore, remain unclear.
These findings point us strongly toward additional clinical questions in immuno-oncology.
Is the ultra-low-dose of 0.3 mg/kg equivalent to currently approved doses? Can these findings be extrapolated to other tumor types?
Besides nivolumab, would similar dose reductions be possible for the other anti–programmed cell death protein 1/programmed death-ligand 1 monoclonal antibodies?
It is evident that similar, ultra-low dosing of atezolizumab is also possible.13 Given the substantial reductions in health care costs with ultra-low-dose immunotherapy, there is an urgent need for near-equivalence studies to further investigate the clinical outcomes associated with dose de-escalation.14
This could greatly increase access to such therapies in resource-poor settings.15
To reduce costs and increase access to immunotherapy, there are fundamentally two strategies: reduce the cost per unit of drug or reduce the amount of drug per patient.
To reduce the unit price on an individual drug, a strong negotiating position would be required on the side of the health care payer.
Alternately, in cases where multiple, substitutable agents are available, prices could be driven down by competition among agents.
Effective price competition may be difficult to achieve, however, as studies have suggested that cancer drug prices often fail to respond to new competition.16–19
The potential for price competition among the programmed cell death protein 1/programmed death-ligand 1 class was further diminished by the FDA decision not to approve sintilimab, which was studied in a phase III trial (ORIENT-11) trial conducted in a Chinese population, citing standards for clinical end points, control arm therapies, and degree of unmet clinical need, which it commonly does not apply to drugs tested in the US setting.20
The drug’s manufacturer had previously stated plans to price sintilimab at approximately 40% below other drugs in the class.21
With limited prospects for lowering the unit prices of immunotherapy drugs, reducing the amount of drug per patient — whether by using lower doses, reduced frequency, or reduced duration of treatment — may be the more immediately accessible option.
The current study of ultra-low dose nivolumab is, therefore, an important step toward realizing this strategy.
This study’s importance extends beyond head and neck cancer and beyond nivolumab.
It demonstrates the potential to successfully execute de-escalation studies despite findings that may run counter to the financial interest of the pharmaceutical industry.
Although much has been written in recent years regarding the extraordinary high cost of cancer drugs, very few viable solutions have been provided.
In most countries, after vigorous lobbying, the status quo has been maintained, and prices remain high.
Here, a very viable solution has been demonstrated and will have significant impact for some patients — saving lives as a direct result of achieving lower drug costs.
Many similar opportunities for dose de-escalation exist for other cancer drugs.
Dose reduction is now well-established as a solution to improve access to abiraterone acetate, a prostate cancer drug.22
These examples should be used as a springboard to assess the opportunities for dose de-escalation across cancer types and pharmaceutical agents.
Many similar opportunities for dose de-escalation exist for other cancer drugs. Dose reduction is now well-established as a solution to improve access to abiraterone acetate, a prostate cancer drug.
In many ways, the current state of cancer immunotherapy is analogous to that of HIV medications two decades ago.
At that time, highly effective medications were available only in wealthy countries.
In Africa, where disease prevalence was high, the drug prices were too high to be widely accessible, and patients continued to die as a result.
Similarly, immunotherapy is highly effective for some cancers, yet it is out of reach for most patients in poor countries.
With lower doses and lower costs, many deaths could be prevented. Perhaps ultra-low dosing of cancer drugs will provide the much-needed solution for these patients.
With lower doses and lower costs, many deaths could be prevented. Perhaps ultra-low dosing of cancer drugs will provide the much-needed solution for these patients.
References
See the original publication
About the authors & affiliations
Aaron P. Mitchell , MD, MPH 1; and Daniel A. Goldstein , MD2,3,4,5
1 Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY
2 Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
3 Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel
4 Clalit Health Service, Tel Aviv-Yafo, Israel
5 Optimal Cancer Care Alliance, Ann Arbor, MI