NEJM
Jonas Hugosson, M.D., Ph.D., Marianne Månsson, Ph.D., Jonas Wallström, M.D., Ph.D., Ulrika Axcrona, M.D., Ph.D., Sigrid V. Carlsson, M.D, Ph.D., M.P.H., Lars Egevad, M.D., Ph.D., Kjell Geterud, M.D., Ph.D., Ali Khatami, M.D., Ph.D., Kimia Kohestani, M.D., Ph.D., Carl-Gustaf Pihl, M.D., Andreas Socratous, M.D., Johan Stranne, M.D., Ph.D., et al., for the GÖTEBORG-2 Trial Investigators*
December 8, 2022
Abstract
BACKGROUND
- Screening for prostate cancer is burdened by a high rate of overdiagnosis.
- The most appropriate algorithm for population-based screening is unknown.
METHODS
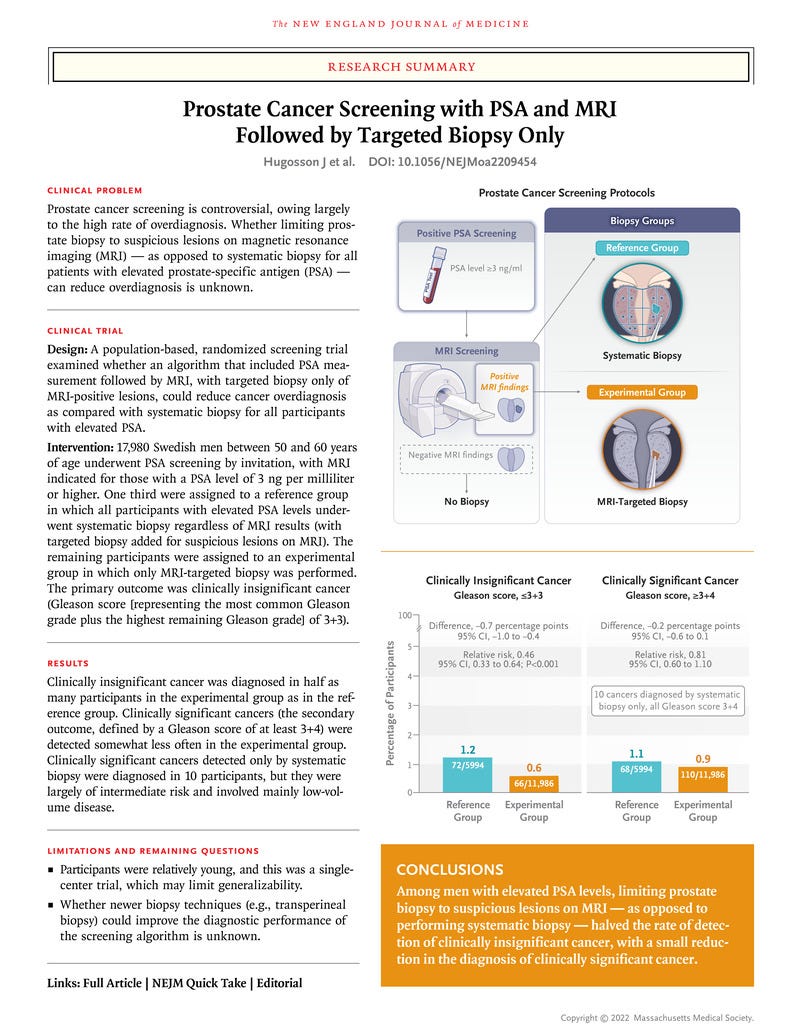
- We invited 37,887 men who were 50 to 60 years of age to undergo regular prostate-specific antigen (PSA) screening.
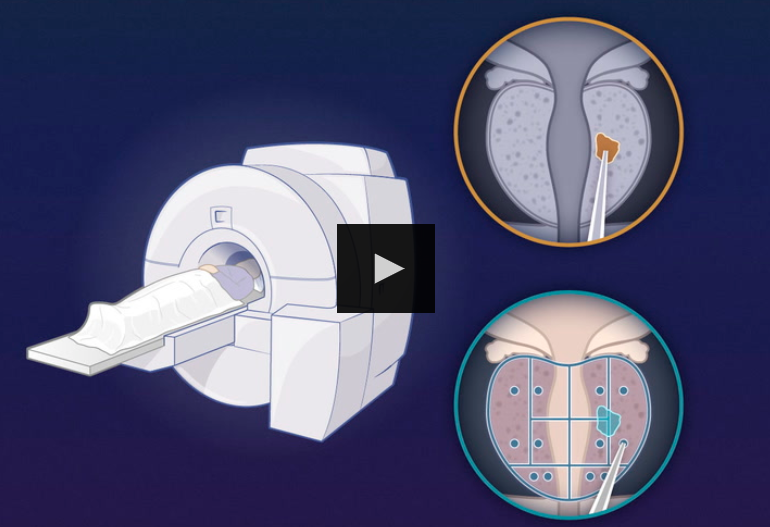
- Participants with a PSA level of 3 ng per milliliter or higher underwent magnetic resonance imaging (MRI) of the prostate;
- one third of the participants were randomly assigned to a reference group that underwent systematic biopsy as well as targeted biopsy of suspicious lesions shown on MRI.
- The remaining participants were assigned to the experimental group and underwent MRI-targeted biopsy only.
- The primary outcome was clinically insignificant prostate cancer, defined as a Gleason score of 3+3.
- The secondary outcome was clinically significant prostate cancer, defined as a Gleason score of at least 3+4.
- Safety was also assessed.
RESULTS
- Of the men who were invited to undergo screening, 17,980 (47%) participated in the trial.
- A total of 66 of the 11,986 participants in the experimental group (0.6%) received a diagnosis of clinically insignificant prostate cancer, as compared with 72 of 5994 participants (1.2%) in the reference group, a difference of −0.7 percentage points (95% confidence interval [CI], −1.0 to −0.4; relative risk, 0.46; 95% CI, 0.33 to 0.64; P<0.001).
- The relative risk of clinically significant prostate cancer in the experimental group as compared with the reference group was 0.81 (95% CI, 0.60 to 1.1).
- Clinically significant cancer that was detected only by systematic biopsy was diagnosed in 10 participants in the reference group; all cases were of intermediate risk and involved mainly low-volume disease that was managed with active surveillance. Serious adverse events were rare (<0.1%) in the two groups.
CONCLUSIONS
- The avoidance of systematic biopsy in favor of MRI-directed targeted biopsy for screening and early detection in persons with elevated PSA levels reduced the risk of overdiagnosis by half …
- … at the cost of delaying detection of intermediate-risk tumors in a small proportion of patients.
(Funded by Karin and Christer Johansson’s Foundation and others; GÖTEBORG-2 ISRCTN Registry number, ISRCTN94604465. opens in new tab.)
Screening for prostate cancer is burdened by a high rate of overdiagnosis.
The avoidance of systematic biopsy in favor of MRI-directed targeted biopsy for screening and early detection in persons with elevated PSA levels reduced the risk of overdiagnosis by half at the cost of delaying detection of intermediate-risk tumors in a small proportion of patients.
Infographic
About the authors & affiliations
Jonas Hugosson, M.D., Ph.D., Marianne Månsson, Ph.D., Jonas Wallström, M.D., Ph.D., Ulrika Axcrona, M.D., Ph.D., Sigrid V. Carlsson, M.D, Ph.D., M.P.H., Lars Egevad, M.D., Ph.D., Kjell Geterud, M.D., Ph.D., Ali Khatami, M.D., Ph.D., Kimia Kohestani, M.D., Ph.D., Carl-Gustaf Pihl, M.D., Andreas Socratous, M.D., Johan Stranne, M.D., Ph.D., et al., for the GÖTEBORG-2 Trial Investigators*
From the Departments of Urology (J.H., A.K., K.K., J.S., R.A.G.), Radiology (J.W., K.G., A.S., M.H.), and Pathology (C.-G.P.), Sahlgrenska University Hospital–Sahlgrenska Academy at Gothenburg University, and the Department of Urology, Sahlgrenska Academy at Gothenburg University (J.H., M.M., S.V.C.), Gothenburg, and the Department of Oncology–Pathology, Karolinska Institute, Stockholm (L.E.) — all in Sweden; the Departments of Pathology and Molecular Oncology, Oslo University Hospital–Radiumhospitalet, Oslo (U.A.); and the Departments of Surgery (Urology Service) and Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York (S.V.C.).
Originally published at https://www.nejm.org.