Consensus Report and Recommendations
Value In Health
December 15, 2021
Yolima Cossio-Gil, MD, MPH
Maisa Omara, BDS, MPH
Carolina Watson, PhD
Joseph Casey, BA
Alexandre Chakhunashvili, PhD
María Gutiérrez-San Miguel, MBA
Pascal Kahlem, PhD
Samuel Keuchkerian, MBA
Valerie Kirchberger, MD
Virginie Luce-Garnier, MD
Dominik Michiels, MSc, QA
Matteo Moro, MD
Barbara Philipp-Jaschek, Mag. phil.
Simona Sancini, MD
Jan Hazelzet, MD, PhD ∗
Tanja Stamm, PhD, Dr. rer. biol. hum., Mag. phil., MSc, MBA
Highlights
- Value-based healthcare aims at improving patient outcomes while optimizing the use of hospitals’ resources among medical personnel, administrations, and support services through an evidence-based, collaborative approach.
- Our blueprint describes how to prepare hospitals for value-based healthcare implementation; analyzes gaps, barriers, and facilitators; and explores the most effective ways to turn patient pathways to a process that results in high-value care.
ABSTRACT
Objectives
Value-based healthcare (VBHC) aims at improving patient outcomes while optimizing the use of hospitals’ resources among medical personnel, administrations, and support services through an evidence-based, collaborative approach. In this article, we present a blueprint for the implementation of VBHC in hospitals, based on our experience as members of the European University Hospital Alliance.
Methods
The European University Hospital Alliance is a consortium of 9 large hospitals in Europe and aims at increasing the quality and efficiency of care to ultimately drive better outcomes for patients.
Results
The blueprint describes how to prepare hospitals for VBHC implementation; analyzes gaps, barriers, and facilitators; and explores the most effective ways to turn patient pathways into a process that results in high-value care.
Using a patient-centric approach, we identified 4 core minimum components that must be established as cornerstones and 7 organizational enablers to waive the barriers to implementation and ensure sustainability.
Conclusion
The blueprint guides through pathway implementation and establishment of key performance indicators in 6 phases, which hospitals can tailor to their current status on their way to implement VBHC.
ORIGINAL PUBLICATION
The Roadmap for Implementing Value-Based Healthcare in European University Hospitals — Consensus Report and Recommendations
Value In Health
December 15, 2021
Yolima Cossio-Gil, MD, MPH et all
Introduction and Background
Value-based healthcare (VBHC) puts patient outcomes at the center of the healthcare process. Instead of purely reimbursing for the services provided, VBHC links outcomes to costs and so determines value. The focus on the value of medical services could be a key element to ensure the sustainability of high quality healthcare systems in the future; moreover, value could continuously drive performance improvement in care.
Thus, VBHC claims for reforming and reconstructing health systems globally: aligning patient pathways and focusing on outcomes can transcend quality, increase efficiency, and enable a patient-centric approach while reducing costs and burden on already overstretched support services.,
Outcomes, which are needed to determine value, must be measured in a standardized manner to become a solid basis for comparison. Such standards exist, but still lack multistakeholder acceptance and large-scale implementation. In addition, we currently do not use the full potential of the collected outcomes data because they are collected in silos, and thus, interoperability, linkage, and access often remain difficult. Moreover, we do not sufficiently collect outcome information directly from patients.
Such data would give us patient-centric insights on the effects of our interventions in the context of routine care and would also uncover patients’ unique experiences of care quality. Several initiatives in Europe are currently tackling some of these issues. Nevertheless, they still act differently on various levels of maturity, and a common road map is missing.
Porter and Lee and Porter and Teisberg who pioneered VBHC argued that the transformation should be based on 6 interrelated elements:
(1) organize into integrated practice units (IPUs),
(2) measure outcomes and costs for every patient,
(3) move to bundled payments for care cycles,
(4) integrate care delivery systems,
(5) expand geographic reach, and
(6) build and enable information technology (IT) platform.
A similar approach has been followed in the so-called Quadruple Aim Model that focuses on increasing population health while reducing the cost per capita and improving the experience of patients and caregivers.
Nevertheless, although the VBHC elements provide a broad view of the systems’ parameters that need to be considered, implementation remains largely in the pilot phase, and hospitals show diverse maturity levels. Thus, a general roadmap of transformational measures toward VBHC is lacking.
In 2017, a total of 9 leading European university hospitals established the European University Hospital Alliance (EUHA), setting out a commitment toward excellence in healthcare, education, and research, with the overall aim to improve the value of care in Europe.
One of EUHA’s working groups focuses specifically on value, with an emphasis on pathways and outcomes.
It was formed to engage in defining the minimum requirements to achieve efficient implementation of VBHC in the environment of university hospitals, assuming that hospitals in the different countries would face similar resources and barriers.
Nevertheless, we also postulated that the initial level of awareness of VBHC in our organizations and the payers’ willingness differed between countries.
Thus, not only similarities but also differences would give EUHA’s working group on value important insights of how barriers could be tackled and which resources could be used.
It is important to point out that starting the process of VBHC could be implemented on different scales, starting from small centers to big, specialized university hospitals personalized to the resources and abilities identified, including also the health system’s perspective and the payers involved.
The Consolidated Framework for Implementation Research (CFIR) provides a theoretical framework for implementing innovations.
It is divided into 5 domains (unadapted or adapted intervention, outer setting, inner setting, individuals involved, and process).
In each domain, the CFIR also suggests systematically assessing potential barriers and facilitators in preparation for the implementation of the proposed innovation.
Therefore, our ambition as a working group was to provide a solid process to implement VBHC based on the 5 CFIR domains.
We wanted to serve the hospital communities in their efforts to ensure that a patient-centric approach is adopted while setting minimum needed requirements to increase efficiency when implementing VBHC measures.
Moreover, we aimed at supporting other hospitals to start, integrate, and further develop VBHC within their institutions and healthcare systems. We also aimed to define the training needs regarding VBHC for different healthcare professionals.
Therefore, we established a generic roadmap (“blueprint”) for the implementation of VBHC in a hospital, including the different phases and the identification of possible enablers and barriers.
Given that there is no single definition of VBHC or the meaning of value in a health context, we used a definition from the European Commission experts.
We also considered that no matter how exact the definition of value was, our proposed blueprint would be of use for organizations in moving toward an outcome-driven patient-centered system.
Design and Participants
We performed an international, multicenter consensus process. The multidisciplinary working group consisted of 2 convenors (Y.C., J.H.), a methodologist (T.S.), and experts in patient-reported health outcomes, care process improvement, and care pathway design. Experts’ backgrounds spanned from nurses, medical doctors, process engineers, statisticians, hospital managers, and outcomes researchers working in 1 of the 9 EUHA university hospitals. An average of 2 experts participated per hospital.
Procedures
The working group members were asked to indicate whether any step was considered, planned, or implemented within their institution. We then met 7 times face to face or virtually over a period of approximately 2 years and added field visits, where possible, separated by periods of 2 months. During the face-to-face meetings at the different hospitals, we analyzed the level of implementation of VBHC, exchanged knowledge on real evidence, and learned from each other. We invited external experts to discuss the following specific topics at our meetings: team collaboration, service design, outcomes measurement, lean methodology and organizational transformation, process improvement, VBHC strategy and tactics, and IT. All experts’ contributions acted on a noncommercial basis. Within the working group meetings, we selected specific critical components for the implementation of VBHC in our hospitals in an iterative manner. We grouped the components into main themes and defined phases of implementation. We then specified for each phase which CFIR domain it addressed.
Definition of the Implementation Process
We drafted a process for implementing VBHC. In email rounds, we asked the working group members to comment on the draft version of the blueprint document until we reached the final version. In case of contradictory comments, the convenors and the methodologist discussed the pros and cons of each argument until consensus was achieved. We circulated the final process within the working group and asked for approval (1 vote per university hospital).
We identified 8 mandatory components to implement VBHC in a hospital ( Fig. 1) and grouped them into 4 main areas: the first 3 areas refer to Porter’s 6 core elements and a fourth one was added by us.
Figure 1 — Analysis of the state of implementation of the 8 core components and 3 additional components of value-based healthcare in the 9 hospitals participating in this study.
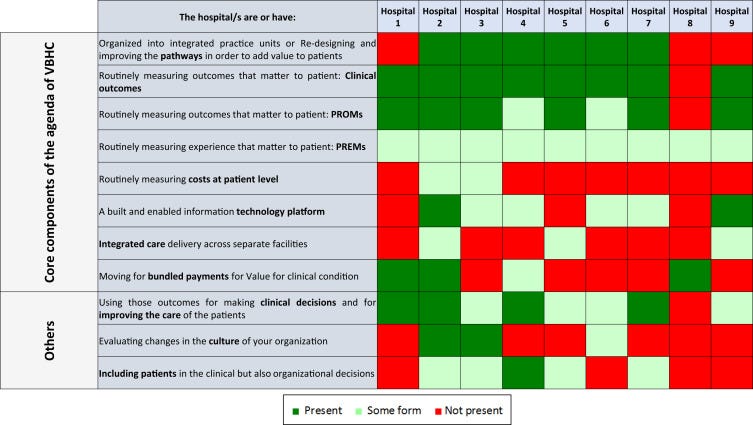
- The first main area refers to (1) organizing care pathways. This is related to the IPUs recommended by Porter.
Implementing a transformation to IPUs can become expensive and time consuming in university hospitals. It also requires extensive organizational and cultural changes in the way care is delivered. Therefore, we recommend starting with redirecting the process of attention in the form of continued assistance by clinical condition. Mutual visits/exchange of staff with participation in care in a previous or subsequent unit can enhance the IPU mindset. Nevertheless, in this article, we consider pathways rather than IPU.
- The second area is (2) collecting a set of outcomes, including clinical outcomes, patient-reported outcomes measures (PROMs) and experience measures (patient-reported experience measures [PREMs]), process indicators, and in a later stage also costs at the patient level.
- (3) Building an information platform is the third area.
We recommend enabling the collection of PROMs integrated within the patient pathway and the visualization of these data using dashboards where indicators are represented. This information platform must allow communication and provide feedback regarding PROMs to clinical teams and also feedback to patients about their own health status. Standardization of outcomes across providers and countries and the interoperability of data sets would lead to comparable and valid results. Different software for collecting the results in between the same hospitals or among them is not a limitation, if the data are harmonized within a common data model. They set up of a data warehouse environment on VBHC in each hospital, taking into account that process indicators and general care information (electronic medical record) need to be linked to clinician-reported outcome measures and PROMs.
- Another fourth area is (4) actively using short-term and long-term outcomes for clinical decisions and for improving care, with the aim for a patient-centric approach.
Cultural change toward actively using the PROMs and PREMs with the patients and enabling shared decision-making tools is mandatory to grasp significant patient-centered care. Although cultural change is probably one of the most difficult to achieve, a potential approach could be to show the new solution’s value for each stakeholder group, including patients and providers. Internal communication strategy, periodical meetings, and messages from formal and informal leaders play an important role in the cultural change.
Based on these 4 main areas, we structured the implementation in 6 development phases ( Fig. 2).
Although phase 1 corresponds to the preparation of the whole organization for VBHC, the after 5 phases entail the concrete implementation of the clinical pathway.
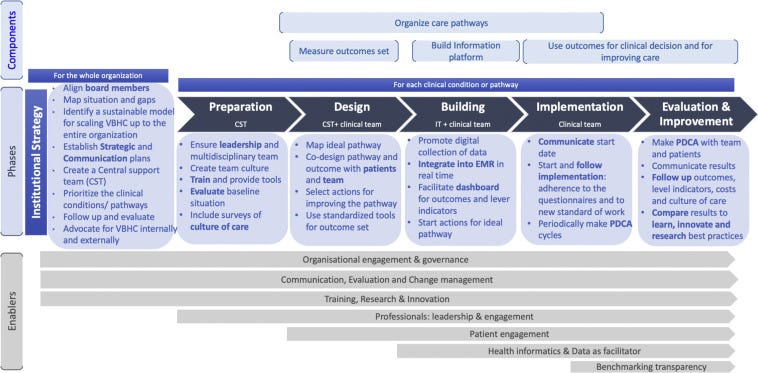
Phase 1: Preparation of the Whole Organization for VBHC: Institutional Strategy (CFIR Domain: Inner Setting)
In this phase, the organization sets up the strategic plan for the implementation of VBHC, including evaluation and follow-up of the maturity and readiness to transform toward VBHC. The main actors in this phase are the board members, who create a strategic umbrella for implementing VBHC in all levels of the organization. If the organization is not yet ready for substantial change, we recommend starting with pilots. In addition, we suggest aligning board members, mapping the current situation, and analyzing the gaps using an evaluation tool of the maturity of the organization about value, for example, the value accelerators. In addition, it is important to develop a sustainable model for scaling VBHC up to the entire organization and consider starting value purchasing methods and negotiating the payment for value. An international, multicenter pilot project has been recently initiated, but results are not yet available. Parallel to this process, the hospital should set up a communication strategy (internal for hospital staff and at management level and external for the general population, the insurance agencies, government/policy makers, and other providers) and advocate for the VBHC inside and outside of the hospital.
A Central Support Team (CST) should be created with at least 1 strategic and 1 operational lead.
They should be trained to be able to help all the clinical leaders with the implementation of each clinical pathway. Staffing these teams is essential. The team size can vary according to the size of the organization. The multidisciplinarity of the support team is recommendable because clinical points of views are essential to connect with the clinical leads and enhance the cultural change but might be “too deep” into the hospital culture. Our experience is that establishing a CST as a central resource is an efficient and less costly measure than to building up this expertise in each clinic. In addition, the CST can learn from previous clinical implementations. New mindsets from other professional profiles may be of added value to bring on fresh inputs in the reorganization of the care pathways that come with VBHC.
Hospitals need to prioritize the clinical conditions/pathways. Board members should decide which one to start with, to apply the appropriate changes at the organizational level.
The best strategy in each specific case should be defined (to go in deep with the pathway or identifying quick wins). We recommend starting with the clinical pathway where clinical leads show a great commitment and the team is engaged. Once the organization is gaining experience in the implementation, other criteria can be added, if there is room for improvement (eg, fragmented care, lack of standardization in the procedures, results, costs). Finally, in this phase, we need to set up a follow-up and evaluate the changes in the organization toward value.
Phase 2: Preparation of Each Clinical Pathway (CFIR Domain: Process)
This phase includes the steps to prepare the team for starting a new working model and for measuring the baseline situation of the costs, team culture, and patient experience before implementing VBHC. The main actors in this phase are the members of the CST. We recommend ensuring leadership and multidisciplinary participation, including physicians, nonphysician health professionals, porters, administrative staff, social care, business intelligence/data managers, pharmacists, and professionals with an economic background, but also collaborations between different disciplines/clinics, for example, in specific meetings such as tumor boards and rehabilitation team meetings. Hospitals should choose leaders and some should sign an agreement of commitment between the clinical lead and the organization. The goals and expectations of the team members must be assessed and potential barriers identified. Team culture should facilitate communication among all the people involved in the clinical pathway and using communication tools should ensure that everyone receives the information. The ideas that arise from people that are not part of the improvement/working group are also included. It is important to ensure their engagement during the process and in decision making.
Hospitals should provide training and tools on methodologies to improve quality and processes, including, but not limited to, shared decision making, a culture of continuous improvement, communication with the patient, and other tools that help the team to lead the changes.
Furthermore, patients should be clustered into rational groups. Organizing care into pathways requires the ability to assign patients to pathways. Sometimes this is straightforward but not always.
We recommend grouping them according to a defined logical model (eg, using certain grouping parameters).
The hospital should evaluate the baseline situation, including the outcomes that are measured, and conduct surveys on the current state of the culture of care.
VBHC is about improving patient treatment results and costs, but also reducing the burden on professionals and improving satisfaction with their work. Therefore, in addition to measuring the baseline costs, PREMs, and the process indicators of each clinical pathway, we recommend measuring the clinical team culture and work environment.
Phase 3: Design (by the CST and the Clinical Team; CFIR Domains: Process and Individuals Involved)
In this phase, the outcome set and the standard of care for patients with the selected clinical condition should be defined.
It should include process indicators to measure over (lead times, reports, presence of key interventions, among others) and the underuse of healthcare, detecting the possible root cause of outcome deficiencies and identifying the appropriateness of clinical practice linked to the outcomes. Surrogate measures, understood as those that are related/associated to higher-level measures, should be taken into account. For instance, in the case of diabetes, hemoglobin A1c can be an example of such a surrogate measure because it has a profound impact on the higher-level measures such as mortality and morbidity (eg, unwanted complications as a result of an intervention because of other clinical conditions).
In this phase, the CST should help the clinical team to find a better standard of work and to decide the outcomes of value that will be measured and monitored.
Important is to codesign the pathway and outcomes with patients and team by considering what matters to patients. Focus groups, journey maps, surveys to patients, and literature reviewing could be some of the methodologies that could help in this stage. A continuous improvement process should be stated based on the outcomes measured.
Phase 4: Building (by the IT and the Clinical Team; CFIR Domains: Settings [Inner and Outer] and Individuals Involved)
In this phase, the IT and business intelligence staff create the solutions for collecting, analyzing, and visualizing the outcomes and the process indicators.
Standardization and interoperability of outcomes and process indicators are essential to ensure that indicators will be comparable, calibrated the same way, and reliable. In parallel, the clinical team should start to implement the main changes to improve the pathway or circuit of attention. Note that the improvement of the pathway also continues in all the other phases in an iterative manner.
Outcome data need to be integrated into the electronic health records. IT experts need to prepare electronic medical records to check key interventions. We recommend moving from retrospective to prospective indicators including costs for an individual patient stay/journey. Dashboards or other tools could help the team to easily visualize indicators of processes and outcomes to make continuous improvement. Visualizing outcomes will help clinicians to improve communication with patients and monitoring in between clinical visits. Patients should have access to their outcomes and evolution over time. In an international, multicenter pilot project, we will use an application to collect patient-reported data.
We will give patients feedback on how they have been doing during a certain period. This information should give patients better opportunities for self-management and improve their relationship with the clinicians. A governance model that ensures the correct handling of all the legal and regulatory matters is a requirement.
Phase 5: Implementing (by the Clinical Teams; CFIR Domains: Process and Individuals Involved)
Implementing VBHC on an organizational level and in a pilot environment requires a multilevel effort.
In this phase, the team should focus on getting the capture of PROMs and the implementation of changes to the healthcare pathway. It should also already at this point consider the continuous monitoring of both. The main actors here are the clinical lead and the clinical team. Measuring outcomes and fostering discussions on treatment pathways will facilitate the culture and organizational changes that were planned in phases 2 and 3 (mainly organizing pathways, breaking profile-based culture and patient-focused culture).
Continuous improvement could be ensured through systematic “Plan Do Check Act” (PDCA) cycles: Follow the implementation process with indicators related to PROM (ie, compliance of the questionnaires) and clinical appropriateness along the pathway (optimal timings, overuse, and underuse). Regular meetings where PDCA could be used as a tool and mindset for improvement, distinguishing PDCA cycles for data collection (ie, involvement of patients and clinicians) and PDCA cycles for the pathway itself.
Phase 6: Evaluation and Improvement (CFIR Domains: Settings [Inner and Outer] and Process)
In this phase, we recommend using the outcomes and process indicators to evaluate the changes and follow-up the improvement.
Annually or biannually, PREMs, a culture within the disease teams and costs, should be assessed and compared with the baseline data. Patient feedback should be used to facilitate continuous improvement. Periodic meetings with the full team (every 2–3 months) to assess the aggregate outcomes and plan actions for improving them. We recommend using a methodology to find the real cause of the problems and to prioritize the actions. Results should be communicated with teams, the board members, the rest of the organization, and external stakeholders according to the strategy defined earlier.
Results could be also compared with learn and innovate. Best practice examples could be identified and used to innovate research and training.
Challenges and Enablers
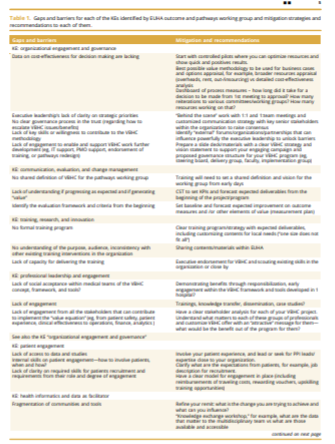
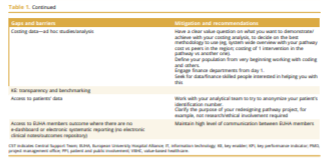
The key gaps and barriers identified by the working group are presented in Table 1. We also propose mitigation strategies and recommendations on how to overcome each of them.
Discussion
This article summarizes the development and implementation of a VBHC blueprint based on the consensus from 9 of the largest university hospitals in the European Union. This was driven by the opinions and experiences of the working group and could be used as a strategic tool and guidance to other university hospitals.
Although previous studies have identified the importance of implementing VBHC, very little has been made in terms of guidelines to healthcare organizations and hospitals as to how implementation should be managed and sustained.
Our roadmap provides for the first time practical and applicable recommendations to each phase of the implementation of VBHC from a hospital management perspective.
With this, we added, in our opinion, an important component to Porter’s agenda.
Moreover, a number of our recommendations relate to patient-centric outcome measurement. Although some countries have reached a certain level of maturity in including patient-reported outcomes in measurement schemes, this is still referred to as cultural change in other countries that are starting to understand the importance of patient-reported outcomes in the healthcare process.
Furthermore, we are currently conducting an international, multicenter pilot project on VBHC, which is specifically targeted to collect patient-reported outcomes on a large scale in 4 European countries to learn from the different experiences.
This article focuses on 3 main points of Porter’s agenda (IPU-outcomes-platform), but it does not address
- the cost-per-patient measurement,
- the organization transformation toward a payment model based on value-based results/value-based pricing,
- or the integration and coordination with other services such as primary care, social care, and others.
Although we address the hospital perspective only, the implementation of VBHC needs to be embedded in a larger healthcare ecosystem.
Implementing VBHC involves a broader range of elements than those described in this report (eg, payments).
For all these reasons, this article should be considered as an initial set of recommendations to foster building the bases at the hospital level, and therefore, future studies on the current topic are recommended.
Although top-down implementation experiences have shown poor response and adherence from patients, because of a lack of commitment to improvement by the lower-level employees, bottom-up experiences are usually pilots with difficulties in terms of consolidation and entrenchment of a general model or blueprint.
From our point of view, hospitals must promote an implementation integrated in the practical clinical practice. The implementation needs the commitment and action of many actors of the healthcare system, beyond the hospital itself.
Clinical teams and patients must have access to the data for making changes at the point of care and discuss the decision with patients.
The hospital cannot change the payment system itself, but can either test different models to convince the payer or get prepared to face the model of the change when it comes.
It is important to understand that implementing VBHC should be based on an iterative process including evidence and a continuous self-learning process to achieve the maximum patient-relevant medical benefits (outcomes) and minimize the costs.
Several challenges have been identified by the task force members at different stages of the iterative consensus process. A cumulative list of them is presented in Table 1.
Although a considerable number of these challenges refers to a lack of understanding and acceptance of the VBHC concept, some of them highlight the need for data to be available to prove the effectiveness of the measures to generate the necessary evidence. ]
Access to standardized outcome data might be a key element in the transition toward VBHC.
Accordingly, it could be more practical to start the process through a well-designed pilot to evaluate risks and opportunities on real-life circumstances. It is recommended to embed the pilot as much as possible in generic systems of the whole hospital and let them grow/mature together, because scaling up the VBHC journey is completely dependent on this balance.
Starting the pilot in a selected health condition or pathway where better circumstances are available (eg, motivated clinical lead and engaged team) could be the way to initiate a proper understanding of the process and how it can be implemented, although it might not reveal at the beginning all challenges that might be encountered.
Moreover, the choice to start with selecting specific health conditions/care pathways or to have a complete VBHC transformation should be tuned to the specific situation of each institution.
Although adopting specific conditions and care pathway strategies and then scaling up to other conditions seem to be a less risky approach, both models should be further studied and explored in the future including their related outcomes and costs.
A limitation of our study is that we only used the European Commission experts’ definition of VBHC.
Further research should focus on international expert consensus studies to better define VBHC considering variations of health systems worldwide. More experts with an economic background should be included and representatives from the payers.
Changes in the structure of facilities of hospitals into an IPU could be costly and require time. If a hospital is not organized into physical IPUs, this should not be seen as a limitation to start the VBHC transformation.
That is why we recommend focusing on and starting care pathways rather than creating a physical IPU.
Once we achieve to measure results and cost, economic experts could analyze the cost-benefit of creating a physical IPU.
Another limitation could have been the initially different level of understanding of VBHC among the working group members. Nevertheless, although this could have been a limitation, it could also be a strength of our study because it allowed us perceiving possible challenges, and it also forced us to question our understanding as a group and go back and reflect on the different preferences and perspectives till reaching consensus. Moreover, we invited external experts to our meetings for reflection and advice. Through these consultations and discussions, we finally achieved a common understanding of the steps which we suggested to implement VBHC.
Identifying a sustainable model for VBHC is a very important approach to visualize the future of VBHC within the organization and ensure the success of the system in the short and long term. Therefore, advocating for VBHC with providers and payers and setting a long-term plan with all stakeholders are an essential step. Hospitals themselves would, in our opinion, also benefit from a new organizational culture that focuses on patient outcomes together with efficient use of resources.
Authors affiliations
Department of Information Systems, Vall d’Hebron, Barcelona Hospital Campus, Barcelona, Spain (Cossio-Gil, Watson, Gutiérrez-San Miguel); Section for Outcomes Research, Center for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna, Austria and Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Vienna, Austria (Omara, Stamm); King’s Health Partners, London, England, UK (Casey); Karolinska University Hospital, Stockholm, Sweden (Chakhunashvili); Scientific Network Management S.L., Barcelona, Spain (Kahlem); CFE-Consulting Group, Saint-Amant-Tallende, France (Keuchkerian); Charité, Berlin, Germany (Kirchberger); Assistance Publique des Hôpitaux de Paris, Paris, France (Garnier); Universitair Ziekenhuis Leuven, Leuven, Belgium (Michiels); Chief Medical Office, Ospedale San Raffaele, Milan, Italy (Moro, Sancini); Chief Medical Office, General Hospital and Medical University of Vienna, Vienna, Austria (Philipp-Jaschek); Erasmus University Medical Center, Rotterdam, The Netherlands (Hazelzet).
Article and Author Information
See the original publication
Acknowledgment
The authors acknowledge and are grateful for the valuable contribution of external organization experts:
Michele Van Der Kemp — Expert on Value-Based Health Care Strategy and Tactics, The Netherlands: Implementation
Muir Gray — Professor and Executive Director at Oxford Centre for Triple Value Healthcare, UK
Gregory Katz — Chaired professor of Innovation and Value in Health at University of Paris School of Medicine, and President of the Consortium VBHC France
Ingeborg Griffioen — Industrial designer at Panton Medical design Agency, The Netherlands — Patient Journey — service design
Mona Krichen — Director of the Organizational transformation engineering department. Agence Nationale d’Appui À la Performance, France
Stéphanie Aftimos — Lean Black Belt — Agence Nationale d’Appui À la Performance, France.
Tim Wilson — Managing Director at Oxford Centre for Triple Value Healthcare, UK
Mathias Ekman — Director Industry Solutions Executive for Healthcare at Microsoft Western Europe, Sweden
Kris Vanhaecht — Associate Professor Quality in Healthcare at KU Leuven, Belgium
François Crémieux, Assistance Publique des Hôpitaux de Paris, France
Originally published at https://www.valueinhealthjournal.com.