Figure 1 | The immune response to SARS-CoV-2 infection.
Determining the duration of protective immunity to infection by SARS-CoV-2 is crucial for understanding and predicting the course of the COVID-19 pandemic.
Clinical studies now indicate that immunity will be long-lasting.
Nature
Andreas Radbruch; Hyun-Dong Chang
14 June 2021
Generating immunity against the SARS-CoV-2 coronavirus is of the utmost importance for bringing the COVID-19 pandemic under control, protecting vulnerable individuals from severe disease and limiting viral spread.
Our immune systems protect against SARS-CoV-2 either through a sophisticated reaction to infection or in response to vaccination.
A key question is, how long does this immunity last?
Writing in Nature, Turner et al. and Wang et al. characterize human immune responses to SARS-CoV-2 infection over the course of a year.
There is ongoing discussion about which aspects of the immune response to SARS-CoV-2 provide hallmarks of immunity (in other words, correlates of immunological protection). However, there is probably a consensus that the two main pillars of an antiviral response are immune cells called cytotoxic T cells, which can selectively eliminate infected cells, and neutralizing antibodies, a type of antibody that prevents a virus from infecting cells, and that is secreted by immune cells called plasma cells. A third pillar of an effective immune response would be the generation of T helper cells, which are specific for the virus and coordinate the immune reaction. Crucially, these latter cells are required for generating immunological memory — in particular, for orchestrating the emergence of long-lived plasma cells, which continue to secrete antiviral antibodies even when the virus has gone.
Immunological memory is not a long-lasting version of the immediate immune reaction to a particular virus; rather, it is a distinct aspect of the immune system. In the memory phase of an immune response, B and T cells that are specific for a virus are maintained in a state of dormancy, but are poised to spring into action if they encounter the virus again or a vaccine that represents it. These memory B and T cells arise from cells activated in the initial immune reaction. The cells undergo changes to their chromosomal DNA, termed epigenetic modifications, that enable them to react rapidly to subsequent signs of infection and drive responses geared to eliminating the disease-causing agent. B cells have a dual role in immunity: they produce antibodies that can recognize viral proteins, and they can present parts of these proteins to specific T cells or develop into plasma cells that secrete antibodies in large quantities. About 25 years ago, it became evident that plasma cells can become memory cells themselves, and can secrete antibodies for long-lasting protection. Memory plasma cells can be maintained for decades, if not a lifetime, in the bone marrow.
The presence in the bone marrow of long-lived, antibody-secreting memory plasma cells is probably the best available predictor of long-lasting immunity. For SARS-CoV-2, most studies so far have analysed the acute phase of the immune response, which spans a few months after infection, and have monitored T cells, B cells and secreted antibodies. It has remained unclear whether the response generates long-lived memory plasma cells that secrete antibodies against SARS-CoV-2.
Turner and colleagues took up the challenge of identifying antibody-secreting memory plasma cells in the bone marrow of people who have recovered from COVID-19 (called convalescent individuals). Memory plasma cells are rare, and those specific for a particular disease-causing agent will obviously be extremely scarce. Nevertheless, Turner and colleagues detected memory plasma cells that secreted antibodies specific for the spike protein encoded by SARS-CoV-2 in 15 of 19 individuals, approximately 7 months after infection. Notably, when the authors obtained samples 4 months later (11 months after SARS-CoV-2 infection), the number of such plasma cells had remained stable in all but one of the individuals analysed. Those plasma cells did not proliferate, which classifies them as bona fide memory plasma cells. Their numbers equalled those of memory plasma cells found in the individuals after vaccination against tetanus or diphtheria, and which provide long-term immunity to those diseases.
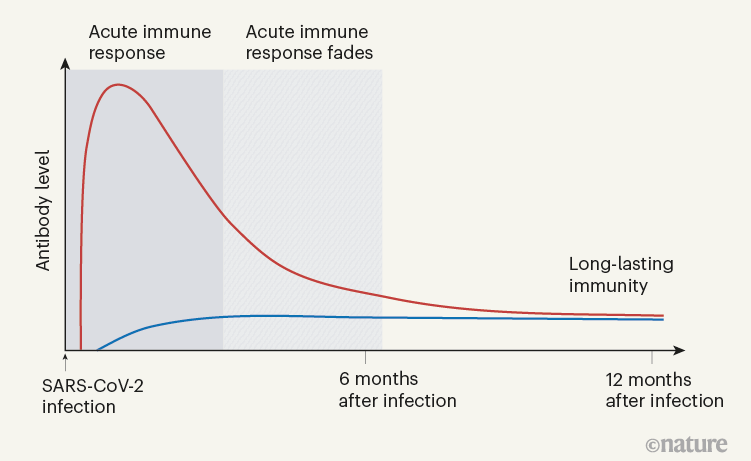
When Turner et al. tracked the concentrations of antibodies against SARS-CoV-2 in the individuals’ blood serum for up to one year, they observed a biphasic pattern (Fig. 1). In the acute immune response around the time of initial infection, antibody concentrations were high. They subsequently declined, as expected, because most of the plasma cells of an acute immune response are short-lived. After a few months, the antibody concentrations levelled off and remained more or less constant at roughly 10–20% of the maximum concentration observed. This is consistent with the expectation that 10–20% of the plasma cells in an acute immune reaction become memory plasma cells, and is a clear indication of a shift from antibody production by short-lived plasma cells to antibody production by memory plasma cells. This is not unexpected, given that immune memory to many viruses and vaccines is stable over decades, if not for a lifetime.
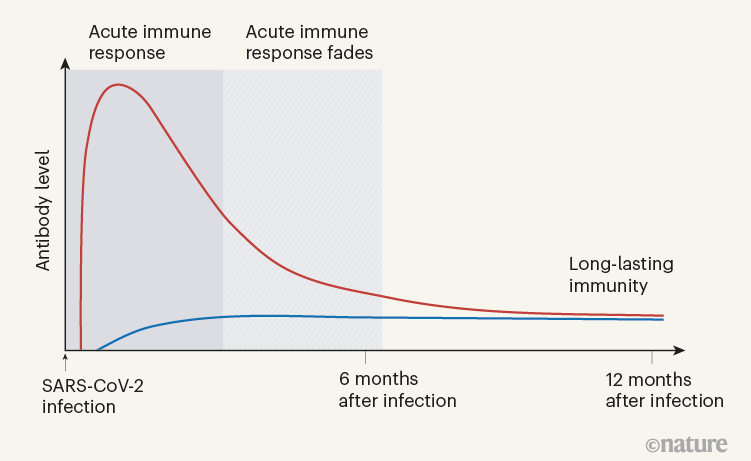
Figure 1 | The immune response to SARS-CoV-2 infection. Data are becoming available that shed light on longer-term aspects of the human immune response to coronavirus infection. One component of the defence response is the production of antibodies that target viral proteins (red line). During the initial, acute phase of the immune response, antibody levels peak rapidly; this peak is generated by short-lived immune cells called plasma cells. Turner et al.1 present clinical evidence, from people who have had COVID-19, that long-lived, memory plasma cells that produce antibodies are generated in the bone marrow. These cells provide long-term antibody production that offers stable protection at a level of 10–20% of that during the acute phase (blue line). Memory plasma cells are a cell type that can be maintained for many years, if not a lifetime8. Wang et al.2 have characterized antibody responses at between six months and a year in people who have been infected with SARS-CoV-2; their results also provide evidence for the generation of immunological memory.
For SARS-CoV, a coronavirus very like SARS-CoV-2 that was originally identified in 2003 and causes severe acute respiratory syndrome (SARS), the continued presence of high concentrations of neutralizing antibodies in blood serum for more than 17 years was reported in 2020. Wang and colleagues’ results suggest that long-term immunity might also be expected for SARS-CoV-2. The authors report a follow-up investigation of serum antibodies and memory B cells specific for SARS-CoV-2 approximately one year after infection. The individuals studied had previously been analysed by Wang and colleagues’ group after six months, but it is only now, after a year, that the transition from an acute immune reaction to the generation of immunological memory has become evident.
Wang et al. show that, between 6 and 12 months after infection, the concentration of neutralizing antibodies remains unchanged. That the acute immune reaction extends even beyond six months is suggested by the authors’ analysis of SARS-CoV-2-specific memory B cells in the blood of the convalescent individuals over the course of the year. These memory B cells continuously enhance the reactivity of their SARS-CoV-2-specific antibodies through a process known as somatic hypermutation. The authors demonstrated this with in vitro tests of antibody neutralization of a broad collection of SARS-CoV-2 variant strains.
Finally, Wang and colleagues show that immunity can be boosted even further in convalescent individuals by vaccinating them after a year. This resulted in the generation of more plasma cells, together with an increase in the level of SARS-CoV-2 antibodies that was up to 50 times greater than before vaccination. Some of the plasma cells will probably be recruited to become memory plasma cells, although this remains to be demonstrated formally, as does the induction of stable, long-term memory as a consequence of SARS-CoV-2 vaccination.
In evaluating vaccine efficacy, we should not expect the high antibody concentrations characteristic of acute immune reactions to be maintained in the memory phase. It is an old misconception, when advocating frequent revaccinations, that antibody concentrations during the acute immune reaction can be compared with those later on, to calculate an imaginary ‘half-life’ of antibody-mediated immunity. This ignores the biphasic character of the immune response (Fig. 1).
The good news is that the evidence thus far predicts that infection with SARS-CoV-2 induces long-term immunity in most individuals. This provides a welcome positive note as we wait for further data on memory responses to vaccination.
About the authors
Andreas Radbruch, is at the German Rheumatism Research Centre Berlin (DRFZ), a Leibniz Institute, Berlin 10117, Germany.
Hyun-Dong Chang, is at the German Rheumatism Research Centre Berlin (DRFZ), a Leibniz Institute, Berlin 10117, Germany.
Competing Interests
The authors declare no competing interests.
References
See the original publication
Originally published at https://www.nature.com.
To download the PDF open the URL below
https://media.nature.com/original/magazine-assets/d41586-021-01557-z/d41586-021-01557-z.pdf
To continue reading:
SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans
Nature,
24 May 2021
Jackson S. Turner; Wooseob Kim, Elizaveta Kalaidina, Charles W. Goss, Adriana M. Rauseo, Aaron J. Schmitz, Lena Hansen, Alem Haile, Michael K. Klebert, Iskra Pusic, Jane A. O’Halloran, Rachel M. Presti & Ali H. Ellebedy
Abstract
Long-lived bone marrow plasma cells (BMPCs) are a persistent and essential source of protective antibodies1–7. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent individuals have a significantly lower risk of reinfection8–10.
Nonetheless, it has been reported that anti-SARS-CoV-2 serum antibodies experience rapid decay in the first few months after infection, raising concerns that long-lived BMPCs may not be generated and humoral immunity against this virus may be short-lived11–13.
Here we demonstrate that in patients who experienced mild infections (n=77), serum anti-SARS-CoV-2 spike (S) antibodies decline rapidly in the first 4 months after infection and then more gradually over the following 7 months, remaining detectable at least 11 months after infection.
Anti-S antibody titers correlated with the frequency of S-specific BMPCs obtained from bone marrow aspirates of 18 SARS-CoV-2 convalescent patients 7 to 8 months after infection.
S-specific BMPCs were not detected in aspirates from 11 healthy subjects with no history of SARS-CoV-2 infection. We demonstrate that S-binding BMPCs are quiescent, indicating that they are part of a long-lived compartment.
Consistently, circulating resting memory B cells directed against the S protein were detected in the convalescent individuals.
Overall, we show that SARS-CoV-2 infection induces a robust antigen-specific, long-lived humoral immune response in humans.
To download the PDF open the link below
https://www.nature.com/articles/s41586-021-03647-4_reference.pdf