health transformation . institute
institute for continuous health transformation
Joaquim Cardoso MSc
Founder and Chief Researcher & Editor
December 20, 2022
This is a republication of the article “AI better than human eye at predicting brain metastasis outcomes”, with the title above.
Source:
News @ York
December 19, 2022
Lassonde engineers create novel technique they hope will provide cancer patients and clinicians with better, faster information
A recent study by York University researchers suggests an innovative artificial intelligence (AI) technique they developed is considerably more effective than the human eye when it comes to predicting therapy outcomes in patients with brain metastases.
The team hopes the new research and technology could eventually lead to more tailored treatment plans and better health outcomes for cancer patients.
“This is a sophisticated and comprehensive analysis of MRIs to find features and patterns that are not usually captured by the human eye,” says York Research Chair Ali Sadeghi-Naini, associate professor of biomedical engineering and computer science in the Lassonde School of Engineering, and lead on the study.
“This is a sophisticated and comprehensive analysis of MRIs to find features and patterns that are not usually captured by the human eye,”
“We hope our technique, which is a novel AI-based predictive method of detecting radiotherapy failure in brain metastasis, will be able to help oncologists and patients make better informed decisions and adjust treatment in a situation where time is of the essence.”
Previous studies have shown that using standard practices, …
… such as MRI imaging — assessing the size, location — and number of brain metastases — well as the primary cancer type and condition of the patient,
… oncologists are able to predict treatment failure (defined as continued growth of the tumour) about 65 per cent of the time.
The researchers created and tested several AI models and their best one had an 83 per cent accuracy.
Previous studies have shown that using standard practices, …oncologists are able to predict treatment failure (defined as continued growth of the tumour) about 65 per cent of the time.
The researchers created and tested several AI models and their best one had an 83 per cent accuracy.
Brain metastases are a type of cancerous tumour that develops when primary cancers in the lungs, breasts, colon or other parts of the body are spread to the brain via the bloodstream or lymphatic system.
While there are various treatment options, stereotactic radiotherapy is one of the more common, with treatment consisting of concentrated doses of radiation targeted at the area with the tumour.
“Not all of the tumours respond to radiation — up to 30 per cent of these patients have continued growth of their tumour, even after treatment,” Sadeghi-Naini says. “This is often not discovered until months after treatment via follow-up MRI.”
“Not all of the tumours respond to radiation — up to 30 per cent of these patients have continued growth of their tumour, even after treatment,” …
“This is often not discovered until months after treatment via follow-up MRI.”
This delay is time patients with brain metastases cannot afford, as it is a particularly debilitating condition with most people succumbing to the disease between three months to five years after diagnosis.
“It’s very important to predict therapy response even before that therapy begins,” Sadeghi-Naini continues.
This delay is time patients with brain metastases cannot afford, as it is a particularly debilitating condition with most people succumbing to the disease between three months to five years after diagnosis.
“It’s very important to predict therapy response even before that therapy begins,”

Using a machine-learning technique known as deep learning, the researchers created artificial neural networks trained on a large pool of data, then taught the AI to pay more attention to specific areas.
“When you look at an MRI, you see areas within or surrounding the tumour where the intensity and pattern is different, so you attend to those parts with your vision system more,” explains Sadeghi-Naini.
“But an AI algorithm is blind to this. The attention mechanism we incorporated into the algorithm helps these AI tools to learn which part of these images are more important and put more weight on that for analysis and prediction.”
The study, now available online, has been published in the IEEE Journal of Translational Engineering in Health and Medicine.
Partially funded by the Terry Fox Research Institute (TFRI), the modelling work was done at Sadeghi-Naini’s lab at York’s Keele Campus with York PhD student Ali Jalalifar, first author on the study.
When it came to data acquisition and interpreting the results from more than 120 patients, the team was able to leverage York’s long-standing collaborative relationship with Sunnybrook Health Sciences Centre in Toronto.
Other funders of the study included the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Hatch Memorial Foundation.
Sadeghi-Naini says that while more research needs to be done, the findings point to AI being a potentially significant tool in precision management of brain metastasis and even other types of cancer down the line.
The next step to adopting this as a clinical practice would be looking at a larger cohort with a multi-institutional data set, from there a clinical trial could be developed.
“If standard treatments can be tailored for patients based on their response to treatments — that can be predicted before treatment even starts — there’s a good chance that the overall survival of the patients can be improved,” he concludes.
The next step to adopting this as a clinical practice would be looking at a larger cohort with a multi-institutional data set, from there a clinical trial could be developed.
As part of its long‐standing program involving the best cancer researchers across Canada, the Terry Fox New Frontiers Program Project Grants, TFRI is funding further research in ultrasound and MRI for cancer therapy by Sadeghi-Naini and a team of clinicians and scientists based out of Sunnybrook Health Sciences Centre to the tune of $6 million over the next six years.
Sadeghi-Naini is leading the biomedical computational-AI core of the program, receiving $900,000 of that funding.
Sadeghi-Naini and a team of clinicians and scientists based out of Sunnybrook Health Sciences Centre to the tune of $6 million over the next six years.
Sadeghi-Naini is leading the biomedical computational-AI core of the program, receiving $900,000 of that funding.
Originally published at https://www.yorku.ca on December 19, 2022.

Names mentioned
York Research Chair and Professor Ali Sadeghi Naini, lead author on the study.
Partially funded by the Terry Fox Research Institute (TFRI), the modelling work was done at Sadeghi-Naini’s lab at York’s Keele Campus with York PhD student Ali Jalalifar, first author on the study.
Other funders of the study included the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Hatch Memorial Foundation.
REFERENCE PUBLICATION

A Self-Attention-Guided 3D Deep Residual Network With Big Transfer to Predict Local Failure in Brain Metastasis After Radiotherapy Using Multi-Channel MRI
SEYED ALI JALALIFAR1 , HANY SOLIMAN2,3,4, ARJUN SAHGAL2,3,4 , AND ALI SADEGHI-NAINI 1,2,3
Abstract:
- A noticeable proportion of larger brain metastases (BMs) are not locally controlled after stereotactic radiotherapy, and it may take months before local progression is apparent on standard follow-up imaging.
- This work proposes and investigates new explainable deep-learning models to predict the radiotherapy outcome for BM.
- A novel self-attention-guided 3D residual network is introduced for predicting the outcome of local failure (LF) after radiotherapy using the baseline treatment-planning MRI.
- The 3D self-attention modules facilitate capturing long-range intra/inter slice dependencies which are often overlooked by convolution layers.
- The proposed model was compared to a vanilla 3D residual network and 3D residual network with CBAM attention in terms of performance in outcome prediction.
- A training recipe was adapted for the outcome prediction models during pretraining and training the down-stream task based on the recently proposed big transfer principles.
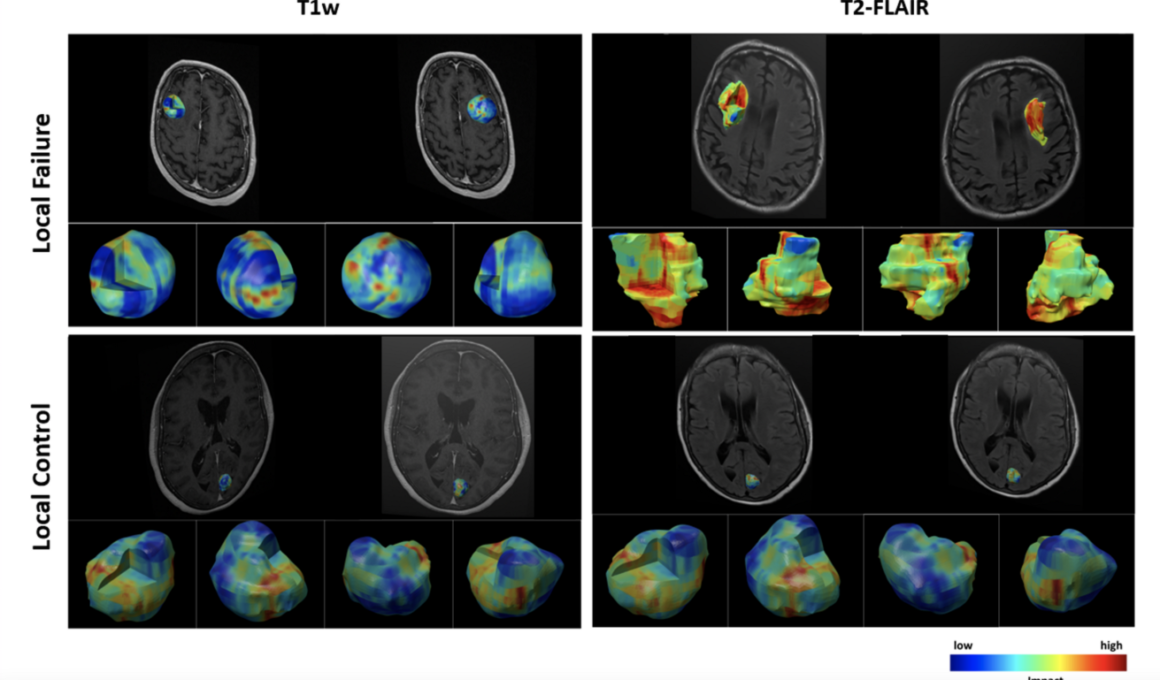
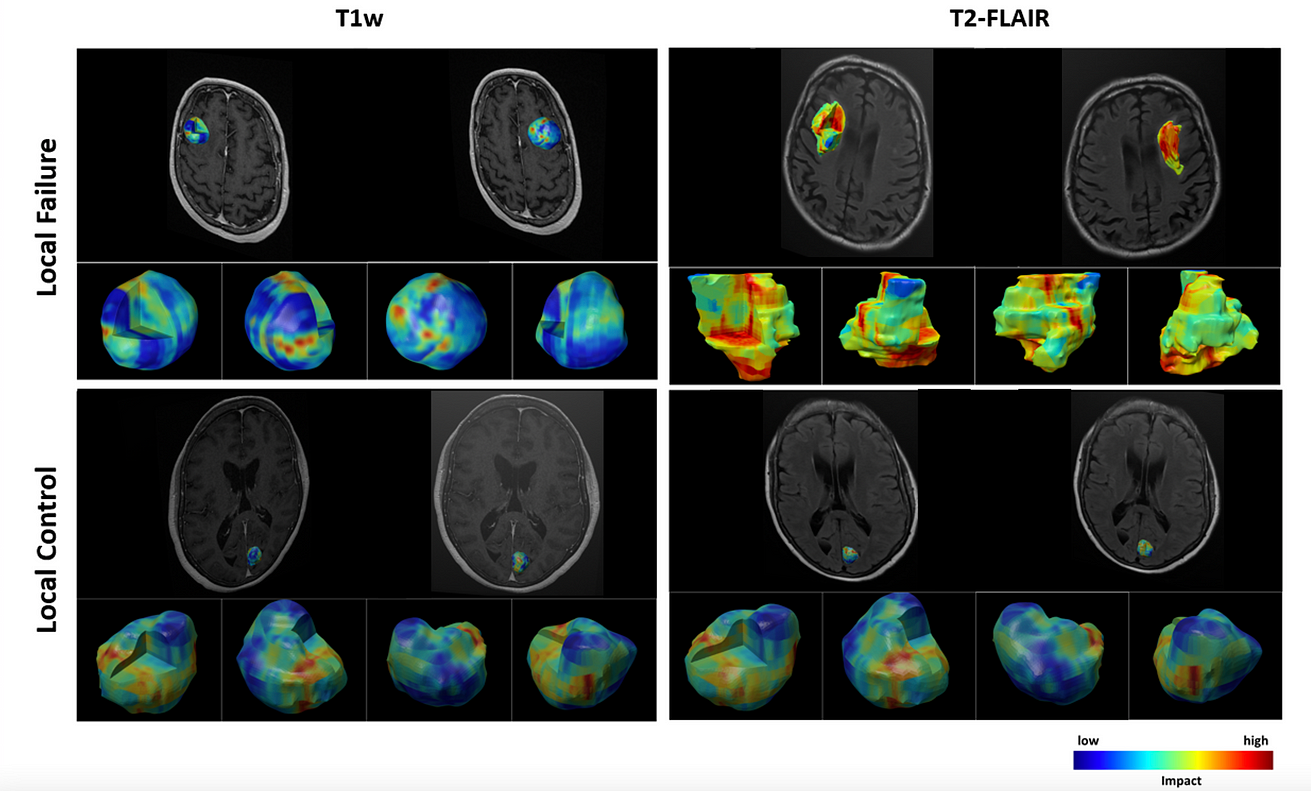
- A novel 3D visualization module was coupled with the model to demonstrate the impact of various intra/peri-lesion regions on volumetric multi-channel MRI upon the network’s prediction.
- The proposed self-attention-guided 3D residual network outperforms the vanilla residual network and the residual network with CBAM attention in accuracy, F1-score, and AUC.
- The visualization results show the importance of peri-lesional characteristics on treatment-planning MRI in predicting local outcome after radiotherapy.
- This study demonstrates the potential of self-attention-guided deep-learning features derived from volumetric MRI in radiotherapy outcome prediction for BM.
- The insights obtained via the developed visualization module for individual lesions can possibly be applied during radiotherapy planning to decrease the chance of LF.
About the authors & affiliations
SEYED ALI JALALIFAR1 , (Member, IEEE), HANY SOLIMAN2,3,4, ARJUN SAHGAL2,3,4 , AND ALI SADEGHI-NAINI 1,2,3, (Senior Member, IEEE)
1Department of Electrical Engineering and Computer Science, Lassonde School of Engineering, York University, Toronto, ON M3J 1P3, Canada
2Physical Sciences Platform, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON M4N 3M5, Canada
3Department of Radiation Oncology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, ON M4N 3M5, Canada
4Department of Radiation Oncology, University of Toronto, Toronto, ON M5T 1P5, Canada
Note from the Editor
It is estimated that between 70,000 to 400,000 new cases of BM are diagnosed each year in the United States [2].
Because of increasing access to neuroimaging and developments in systemic therapies for patients with metastatic disease, as well as increased physician and patient awareness of BMs, the incidence of BM is expected to increase among cancer patients [1].