the health transformation . institute
research institute & knowledge portal
Joaquim Cardoso MSc*
Chef Strategy Officer (CSO), Chief Researcher and Editor
November 23, 2022
MSc* from London Business School — MIT Sloan Masters Program
Executive Summary (source: The Lancet)
What is the message?
- From an estimated 13·7 million (95% UI 10·9–17·1) infection-related deaths in 2019, there were 7·7 million deaths (5·7–10·2) associated with the 33 bacterial pathogens (both resistant and susceptible to antimicrobials) across the 11 infectious syndromes estimated in this study.
- We estimated deaths associated with the 33 bacterial pathogens to comprise 13·6% (10·2–18·1) of all global deaths and 56·2% (52·1–60·1) of all sepsis-related deaths in 2019.
- Five leading pathogens — Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa — were responsible for 54·9% (52·9–56·9) of deaths among the investigated bacteria.
- Three syndromes are responsible for more than 75% of the estimated 7·7 million bacteria-related deaths that occurred in 2019.
- Lower respiratory infections, bloodstream infections, and peritoneal and intra-abdominal infections would rank as the third, seventh, and 13th leading causes of death globally for 2019, respectively, all ahead of other causes such as HIV, colorectal cancer, or self-harm.
What is this important?
- In 2019, more than 6 million deaths occurred as a result of three bacterial infectious syndromes, with lower respiratory infections and bloodstream infections each causing more than 2 million deaths and peritoneal and intra-abdominal infections causing more than 1 million deaths.
- Compared with GBD Level 3 underlying causes of death, deaths associated with these bacteria would rank as the second leading cause of death globally in 2019; hence, they should be considered an urgent priority for intervention within the global health community.
What are the recommendations?
- Strategies to address the burden of bacterial infections include infection prevention, optimised use of antibiotics, improved capacity for microbiological analysis, vaccine development, and improved and more pervasive use of available vaccines.
- These estimates can be used to help set priorities for vaccine need, demand, and development.
What are the strategies?
The 33 bacterial agents investigated as part of this study comprise a significant cause of health loss globally, and strategies to address this substantial burden cover a wide range of interventions.
- First, infection prevention is the foundation to reducing the burden of infections
- Second, vaccination can have a substantial effect on the burden of bacterial infections through a number of routes
- Third, availability of basic acute care services can reduce the number of deaths associated with these bacterial infections
- Finally, a strategic approach and ample investment in the development of new and effective antibiotics are essential to face the increasing threat posed by bacterial antimicrobial resistance and bacterial infections in general.
In Summary
The analyses show that bacterial infections are a clinically significant cause of health loss globally.
- Five pathogens were each involved in more than 500 000 deaths in 2019: S aureus, E coli, S pneumoniae, K pneumoniae, and P aeruginosa.
- Three infectious syndromes, each responsible for more than 1 million deaths in 2019, comprised more than 75% of deaths due to bacterial infections.
- A sobering reality is that a high burden of treatable infections occurred in very young age groups.
- Building stronger health systems with more robust diagnostic infrastructure, improved diagnostic imaging and microbiological capacity, and standardised workflows are crucial steps to address this substantial burden, together with implementing appropriate infection control and antimicrobial stewardship measures.
- Essential prevention strategies include improved access to safe drinking water and sanitation facilities, increased rates of vaccination, new vaccine development, and improving access to the appropriate antibiotic for an infection.
- There is a need to reconcile the right to antimicrobial access with non-judicious use, particularly with regard to expensive and newer generation antimicrobials.
- Predictive mathematical modelling and further advancements in genomic epidemiology of infections will increase insights at the global level to understand pathogens’ evolution, epidemiology, and pathogenesis, and will better inform future approaches.
Infographic
Figure 1 — Global number of deaths (A) and YLLs (B), by pathogen and infectious syndrome, 2019
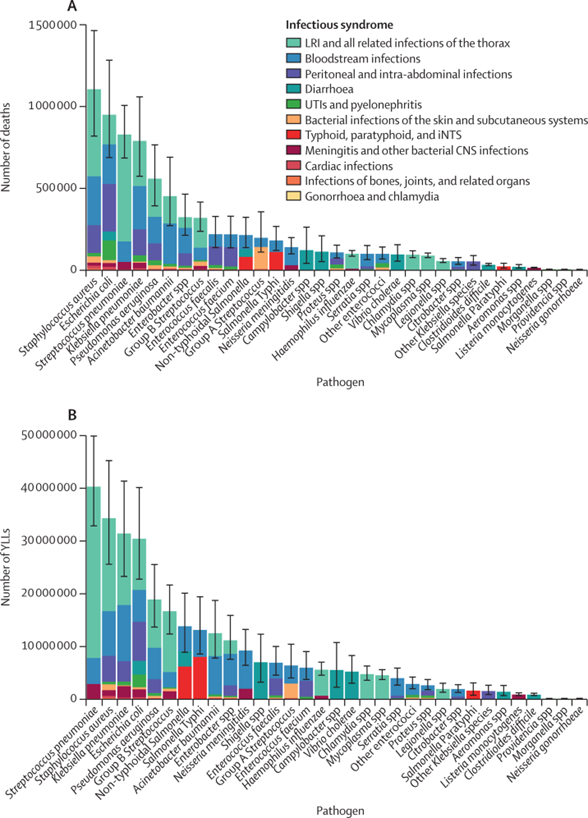
Figure 2 — Overall age-standardised mortality rate per 100 000 population for 33 pathogens investigated, 2019
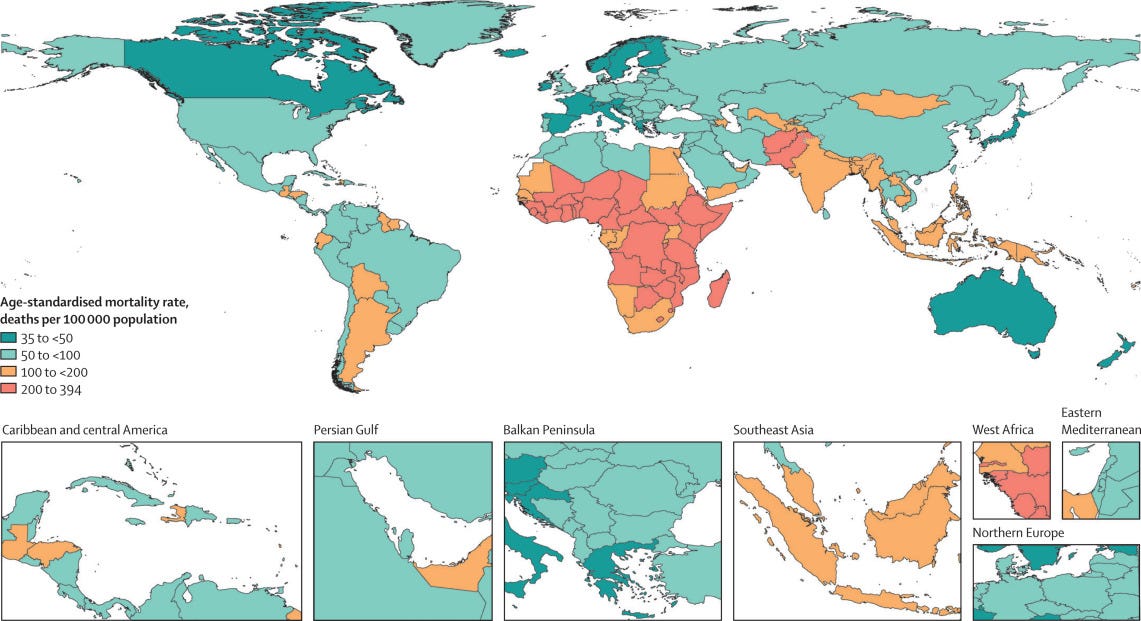
Figure 3- Pathogen responsible for the highest age-standardised mortality rate per 100 000 population (A) and for the highest age-standardised YLL rate per 100 000 population (B), for each country or territory, 2019
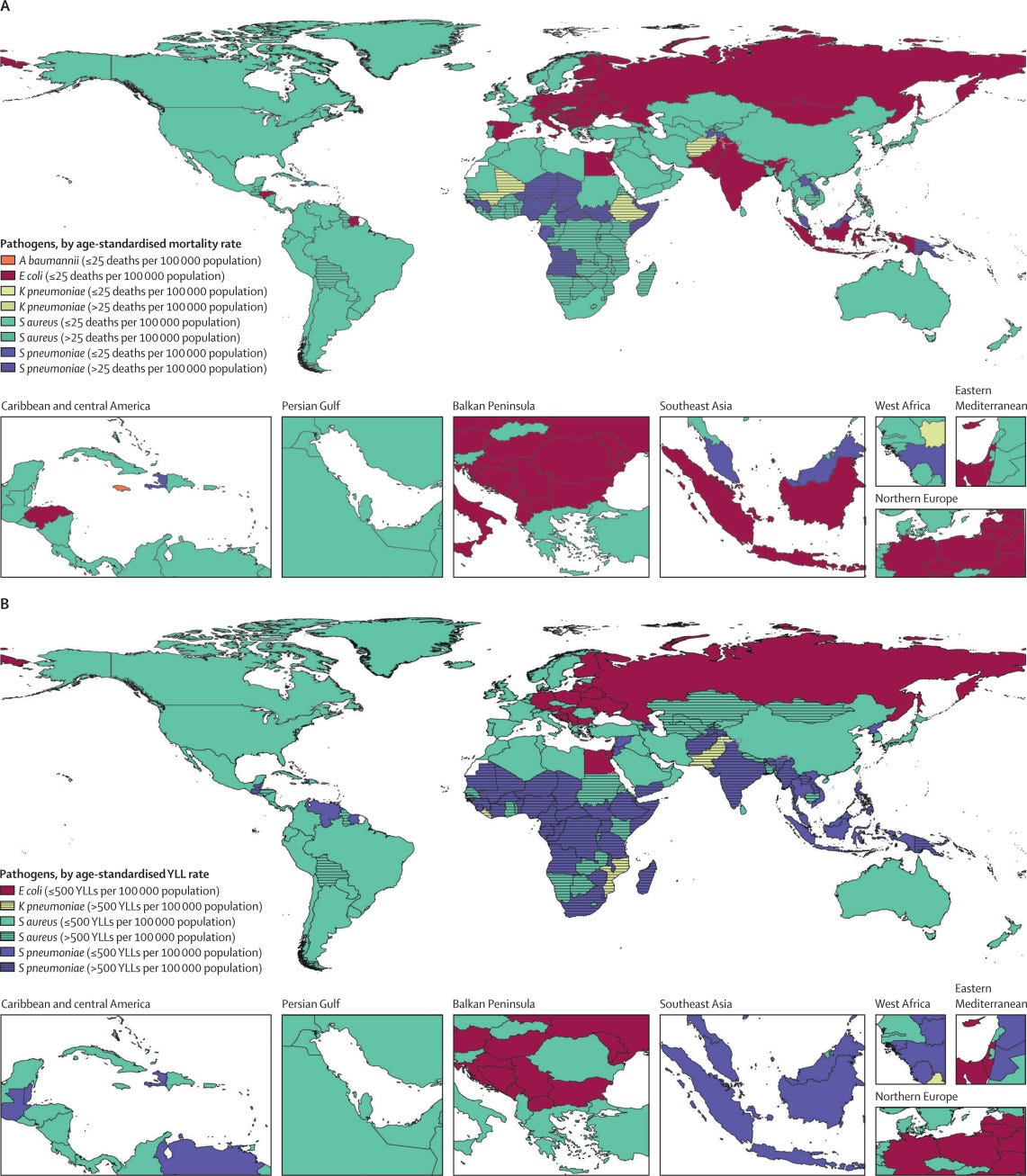
Figure 4 — Global number of deaths (A) and YLLs (B), by pathogen and GBD super-region, 2019
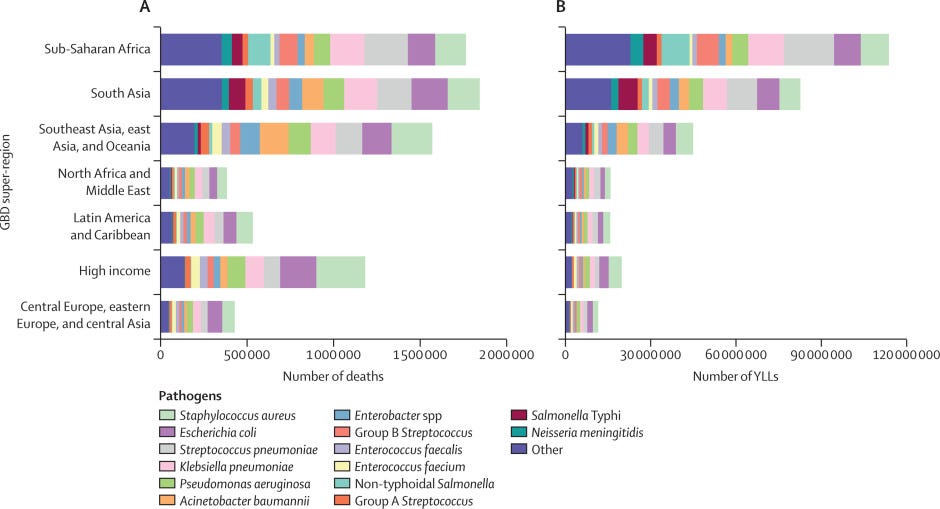
Figure 5 — Global number of deaths, by pathogen, age, and sex groups, 2019
ORIGINAL PUBLICATION (the lancet) — excerpt

Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the GBD Study 2019
The Lancet
GBD 2019 Antimicrobial Resistance
Summary
Background
- Reducing the burden of death due to infection is an urgent global public health priority.
- Previous studies have estimated the number of deaths associated with drug-resistant infections and sepsis and found that infections remain a leading cause of death globally.
- Understanding the global burden of common bacterial pathogens (both susceptible and resistant to antimicrobials) is essential to identify the greatest threats to public health.
- To our knowledge, this is the first study to present global comprehensive estimates of deaths associated with 33 bacterial pathogens across 11 major infectious syndromes.
Methods
- We estimated deaths associated with 33 bacterial genera or species across 11 infectious syndromes in 2019 using methods from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, in addition to a subset of the input data described in the Global Burden of Antimicrobial Resistance 2019 study.
- This study included 343 million individual records or isolates covering 11 361 study-location-years.
- We used three modelling steps to estimate the number of deaths associated with each pathogen: deaths in which infection had a role, the fraction of deaths due to infection that are attributable to a given infectious syndrome, and the fraction of deaths due to an infectious syndrome that are attributable to a given pathogen.
- Estimates were produced for all ages and for males and females across 204 countries and territories in 2019.
- 95% uncertainty intervals (UIs) were calculated for final estimates of deaths and infections associated with the 33 bacterial pathogens following standard GBD methods by taking the 2·5th and 97·5th percentiles across 1000 posterior draws for each quantity of interest.
Findings
- From an estimated 13·7 million (95% UI 10·9–17·1) infection-related deaths in 2019, there were 7·7 million deaths (5·7–10·2) associated with the 33 bacterial pathogens (both resistant and susceptible to antimicrobials) across the 11 infectious syndromes estimated in this study.
- We estimated deaths associated with the 33 bacterial pathogens to comprise 13·6% (10·2–18·1) of all global deaths and 56·2% (52·1–60·1) of all sepsis-related deaths in 2019.
From an estimated 13·7 million (95% UI 10·9–17·1) infection-related deaths in 2019, there were 7·7 million deaths (5·7–10·2) associated with the 33 bacterial pathogens (both resistant and susceptible to antimicrobials) across the 11 infectious syndromes estimated in this study.
- Five leading pathogens — Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa — were responsible for 54·9% (52·9–56·9) of deaths among the investigated bacteria.
- The deadliest infectious syndromes and pathogens varied by location and age.
- The age-standardised mortality rate associated with these bacterial pathogens was highest in the sub-Saharan Africa super-region, with 230 deaths (185–285) per 100 000 population, and lowest in the high-income super-region, with 52·2 deaths (37·4–71·5) per 100 000 population.
- S aureus was the leading bacterial cause of death in 135 countries and was also associated with the most deaths in individuals older than 15 years, globally.
- Among children younger than 5 years, S pneumoniae was the pathogen associated with the most deaths.
- In 2019, more than 6 million deaths occurred as a result of three bacterial infectious syndromes, with lower respiratory infections and bloodstream infections each causing more than 2 million deaths and peritoneal and intra-abdominal infections causing more than 1 million deaths.
Five leading pathogens — Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa — were responsible for 54·9% (52·9–56·9) of deaths among the investigated bacteria.
In 2019, more than 6 million deaths occurred as a result of three bacterial infectious syndromes, with — (1) lower respiratory infections and (2) bloodstream infections each causing more than 2 million deaths and peritoneal and (3) intra-abdominal infections causing more than 1 million deaths.
Interpretation
- The 33 bacterial pathogens that we investigated in this study are a substantial source of health loss globally, with considerable variation in their distribution across infectious syndromes and locations.
- Compared with GBD Level 3 underlying causes of death, deaths associated with these bacteria would rank as the second leading cause of death globally in 2019; hence, they should be considered an urgent priority for intervention within the global health community.
- Strategies to address the burden of bacterial infections include infection prevention, optimised use of antibiotics, improved capacity for microbiological analysis, vaccine development, and improved and more pervasive use of available vaccines.
- These estimates can be used to help set priorities for vaccine need, demand, and development.
Funding
- Bill & Melinda Gates Foundation, Wellcome Trust, and Department of Health and Social Care, using UK aid funding managed by the Fleming Fund.
Introduction
Communicable diseases have long been recognised as a cause of substantial health loss globally, but few studies to date have concentrated on pathogen-specific mortality caused by common bacterial pathogens.
Many estimates exist for pathogens like Mycobacterium tuberculosis, Plasmodium spp, and HIV but estimates of the burden of bacterial infections have been restricted to either a small number of locations, specific populations (such as invasive pneumococcal disease in children younger than 5 years), or a small number of bacteria in the context of the scope of infectious syndromes (eg, Streptococcus pneumoniae and Neisseria meningitidis as a cause of meningitis). The US Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance and Emerging Infections Program, and the European CDC’s European Antimicrobial Resistance Surveillance Network have provided crucial estimates of selected invasive bacterial infections in high-income countries. These estimates are important first steps in building our understanding of the burden of specific bacterial infections but they provide an incomplete picture: within the locations with the greatest infectious burden, the mortality associated with these pathogens remains unknown, making it difficult to set global public health priorities.
Added value of this study
To our knowledge, this is the first study to produce global estimates of mortality associated with 33 clinically significant bacterial pathogens (including those susceptible to antibacterial compounds) across 11 infectious syndromes, and to provide these estimates for all ages and for males and females across 204 countries and territories in 2019.
This analysis is intended to provide an audit of the mortality associated with common bacterial pathogens. We estimated the number of deaths associated with each of these bacterial pathogens using three modelling steps: deaths where infection had a role, the fraction of deaths due to infection attributable to a given infectious syndrome, and the fraction of deaths due to infectious syndromes attributable to a given pathogen. Deaths in which infection had a role were estimated using the number of deaths for which either the underlying cause of death was infectious or the pathway of death was through sepsis. The fraction of deaths due to infection attributable to a given infectious syndrome was estimated using data to determine the infectious syndrome responsible for sepsis by underlying cause of death, age, sex, and geographical location. The fraction of deaths due to an infectious syndrome attributable to a given pathogen was estimated by integrating estimates of pathogen-specific and syndrome-specific case-fatality ratios with modelled pathogen distributions for each infectious syndrome that varied by age and geographical location.
Implications of all the available evidence
Our findings show that more than half of all global bacterial deaths in 2019 were due to five bacterial pathogens: Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
The substantial burden of health loss associated with these five pathogens requires increased attention from the global health community and collaborative intervention approaches.
Understanding the leading infectious syndromes and pathogens for each region is of the utmost importance so that targeted prevention efforts can be implemented.
This study can be used to guide strategies for reducing the burden of bacterial infectious diseases, including infection prevention and control measures, vaccine development and implementation, and the availability of basic acute care services.
… more than half of all global bacterial deaths in 2019 were due to five bacterial pathogens: Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
This study can be used to guide strategies for reducing the burden of bacterial infectious diseases, including infection prevention and control measures, vaccine development and implementation, and the availability of basic acute care services.

Introduction
Communicable diseases have long been highlighted as a global public health priority and are recognised as a leading cause of health loss globally., ,
A recent study estimated that there were more than 10 million sepsis-related deaths in 2017, indicating that infections were involved in more than 20% of deaths globally for that year.
A recent study estimated that there were more than 10 million sepsis-related deaths in 2017, indicating that infections were involved in more than 20% of deaths globally for that year.
Reducing the number of deaths due to infections is a foundational principle in moving towards health equity because there is a disproportionate infectious burden in low-income and middle-income countries (LMICs).,
Preventing and effectively treating infections is also essential to achieving Sustainable Development Goal (SDG) 3:
ensure healthy lives and promote wellbeing for all at all ages. Although the contribution of non-bacterial causes (eg, fungal infections, malaria, and HIV) to the overall infection burden must be acknowledged, reducing the number of cases and health impact of bacterial infectious diseases is a priority area that necessitates a multipronged approach with infection prevention and control measures; vaccine development, deployment, and uptake; and early and effective case management.,
Detailed estimates of the number of deaths related to bacterial infections and their causes are an important step in tracking progress towards global health goals and are essential to inform priorities for vaccine and drug development.
To date, no global burden estimates exist for many common bacterial pathogens, making establishment of public health priorities difficult.
The few estimates that do exist are often constrained to specific pathogens, infectious syndromes, or high-income countries.
For example, global estimates of the burden of Streptococcus pneumoniae are available; however, these estimates are mostly restricted to children younger than 5 years or as a cause of pneumonia or meningitis and do not reflect the total burden across all populations and all infectious syndromes. Estimates of selected invasive bacterial infections exist in high-income countries that use passive surveillance systems, such as the US Centers for Disease Control (CDC) Active Bacterial Core surveillance and Emerging Infections Program and the European CDC’s European Antimicrobial Resistance Surveillance Network.
Although such estimates offer important insights, no comprehensive estimates exist covering all locations for a broad range of bacteria across major infectious syndromes.
Notably absent are country-level estimates for LMICs, which have the greatest burden of infectious diseases, as also emphasised by the recent Global Burden of Antimicrobial Resistance 2019 study.
For this reason, there has been profound neglect of these pathogens, and relevant infectious syndromes, in global advocacy campaigns aiming to maximise life-saving interventions.
In this study, we present, to our knowledge, the first global estimates of deaths associated with 33 clinically significant bacterial pathogens (both susceptible and resistant to antimicrobials), across 11 infectious syndromes in 2019.
We used data obtained from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 and the Global Burden of Antimicrobial Resistance 2019 study to estimate global, regional, and national mortality and years of life lost (YLLs) associated with these 33 bacterial pathogens across 204 countries and territories and 286 underlying causes of death, by age and sex, in 2019.
This manuscript was produced as part of the GBD Collaborator Network and in accordance with the GBD Protocol.
Methods & Other Sections
See the original publication (this is an excerpt version only)

Discussion
To our knowledge, this is the first study to provide global estimates on mortality and YLLs for a wide range of bacterial genera and species across 11 major infectious syndromes.
We found that, collectively, the 33 analysed bacteria were associated with 7·7 million (95% UI 5·7–10·2) deaths in 2019, with an all-age mortality rate of 99·6 deaths (74·2–132) per 100 000 population.
These bacteria were involved in 13·6% (10·1–18·1) of global deaths in 2019 and, compared with Level 3 GBD underlying causes of death, would rank as the second leading cause of death globally, behind ischaemic heart disease.
… compared with Level 3 GBD underlying causes of death, would rank as the second leading cause of death globally, behind ischaemic heart disease.
Individually, four pathogens were associated with more than 750 000 deaths and 30 million YLLs globally in 2019, and their ranks as leading Level 3 causes of death in 2019 would be as follows:
S aureus would rank as the 15th, E coli as the 18th, S pneumoniae as the 20th, and K pneumoniae as the 21st leading Level 3 cause of death.
There was considerable variation in the burden of bacterial infections, with the greatest number of deaths occurring in the sub-Saharan Africa super-region, where we clearly saw the effect of both Gram-positive and Gram-negative pathogens.
The disparate burden in sub-Saharan Africa is magnified by the substantial YLL burden associated with these bacteria in this super-region compared with other super-regions.
Individually, four pathogens were associated with more than 750 000 deaths and 30 million YLLs globally in 2019, and their ranks as leading Level 3 causes of death in 2019 would be as follows:
S aureus would rank as the 15th, E coli as the 18th, S pneumoniae as the 20th, and K pneumoniae as the 21st leading Level 3 cause of death.
By estimating mortality and YLLs for a broad range of pathogens and infectious syndromes, we have produced a global account of bacteria for which the burden was previously unknown and, perhaps, underappreciated.
More than half of bacterial deaths in our study were caused by one of five pathogens: S aureus, E coli, S pneumoniae, K pneumoniae, and P aeruginosa.
More than half of bacterial deaths in our study were caused by one of five pathogens: S aureus, E coli, S pneumoniae, K pneumoniae, and P aeruginosa.
Of these pathogens, only S pneumoniae has been the focus of global surveillance and public health initiatives.,
Although infectious diseases like HIV/AIDS, tuberculosis, and neglected tropical diseases each have their own SDG indicators (eg, SDG 3.3) and have substantial global public health investment (eg, The Global Fund to Fight AIDS, Tuberculosis and Malaria), the bacterial pathogens we found to be associated with a greater fatal burden are not a major focus of any global public health initiatives.
Of these pathogens, only S pneumoniae has been the focus of global surveillance and public health initiatives.,
Only recently have there been calls to expand the scope of The Global Fund to include more common bacteria, although in the context of antimicrobial resistance.
S aureus was the leading bacterial pathogen in most countries and was the only pathogen associated with more than 1 million deaths and 34 million YLLs globally, yet there is no global public health investment directed at S aureus.
S aureus was the leading bacterial pathogen in most countries and was the only pathogen associated with more than 1 million deaths and 34 million YLLs globally, — yet there is no global public health investment directed at S aureus.
Instead, S aureus is included in surgical site infection prevention and antimicrobial resistance initiatives, Global priorty list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.
World Health Organization, 2017. which focus on methicillin-resistant S aureus (known as MRSA), despite the fact that strains with such resistance comprise only a subset of the S aureus burden.
Although WHO prioritised S aureus in 2014 as one of the seven bacteria of international concern, this was in the context of antimicrobial resistance and little has been done regarding the susceptible S aureus burden.
Similarly, E coli and K pneumoniae are collectively associated with more deaths and YLLs than S pneumoniae or tuberculosis, yet they receive comparatively little public health attention relative to their burden, and minimal research funding relative to other diseases with a comparable, or lower, burden.
A 2020 analysis of global funding for infectious disease research found that HIV research was awarded US$42 billion in funding compared with $1·4 billion for research on Staphylococcus spp and $800 million for E coli research over the same period (between 2000 and 2017).
A 2020 analysis of global funding for infectious disease research found that HIV research was awarded US$42 billion in funding compared with $1·4 billion for research on Staphylococcus spp and $800 million for E coli research over the same period (between 2000 and 2017).
The investments in HIV research are certainly warranted and, although bacterial infections could be tackled with different overlapping strategies, this disparity in funding might have been driven, in part, by the shortage of global burden numbers for these bacterial pathogens.
The 33 bacterial agents investigated as part of this study comprise a significant cause of health loss globally, and strategies to address this substantial burden cover a wide range of interventions.
- First, infection prevention is the foundation to reducing the burden of infections
- Second, vaccination can have a substantial effect on the burden of bacterial infections through a number of routes
- Third, availability of basic acute care services can reduce the number of deaths associated with these bacterial infections
- Finally, a strategic approach and ample investment in the development of new and effective antibiotics are essential to face the increasing threat posed by bacterial antimicrobial resistance and bacterial infections in general.
First, infection prevention is the foundation to reducing the burden of infections.
Infection prevention broadly includes in-hospital programmes aimed at reducing hospital-acquired infection, and community programmes that focus on health education, management of malnutrition in LMICs, and the core principles of access to clean water, sanitation, and hygiene.,
Second, vaccination can have a substantial effect on the burden of bacterial infections through a number of routes.
Implementation and uptake of vaccines for bacteria like S pneumoniae can directly reduce the burden of bacterial infections, and new generations of vaccines will target older age groups that we have found are significantly affected by this bacterial agent.
Beyond this, uptake of vaccination for non-bacterial infections like influenza, where bacterial superinfection is a common complication, can also reduce the burden of bacterial infections.
Additionally, vaccine development is crucial for bacteria for which no vaccine exists, and these estimates could help set vaccine development priorities.
However, issues on how to tackle the bacteria that can be present as commensal microbiota have to be considered.
For example, the alteration of commensal bacteria can influence susceptibility to gastrointestinal diseases, which might be an issue when developing a vaccine against E coli; different biology and vaccinology approaches hold the promise of resolving this conundrum.
Third, availability of basic acute care services can reduce the number of deaths associated with these bacterial infections.
Such services include timely access to appropriate antibiotics, microbiological capacity to identify the responsible pathogen of an infection, and provision of supportive care.,
Finally, a strategic approach and ample investment in the development of new and effective antibiotics are essential to face the increasing threat posed by bacterial antimicrobial resistance and bacterial infections in general.
Effective antimicrobials exist for all 33 of the investigated bacteria, yet much of the disproportionately high burden in LMICs might be attributable to inadequate access to effective antimicrobials, weak health systems, and insufficient prevention programmes.[The impact of water and sanitation on diarrhoeal disease burden and over-consumption of antibiotics. London School of Economics and Political Science, March, 2016.]
Effective antimicrobials exist for all 33 of the investigated bacteria, yet much of the disproportionately high burden in LMICs might be attributable to inadequate access to effective antimicrobials, weak health systems, and insufficient prevention programmes.,
Many barriers to accessing these effective antimicrobials have been described.
First, health-care-seeking behaviours are impeded by high out-of-pocket costs, driven by deficiencies in government funding for health and unaffordable drug prices in LMICs.
Second, unwarranted antibiotic use caused by poor education of health-care providers, regulatory issues, self-medication, and restricted availability of antibiotics can lead to the wrong antimicrobial being prescribed, which, if too broad, can promote resistance and, if ineffective, risks progression of infection.
Third, unstable supply chains and poor quality control can result in the desired antibiotic being unavailable or the dissemination of substandard or counterfeit antimicrobials to the consumer.
Improving access to antibiotics requires a nuanced and location-specific response because ease of access must be weighed against risk of antibiotic overuse (a problem compounded by the issue of self-medication in LMICs), which contributes to the increase in antimicrobial resistance.
Furthermore, the use of antibiotics in animal husbandry must be taken into account.
In this study, we addressed the overall burden of infections both susceptible and resistant to antimicrobials, but our previous study highlighted the issue of resistance and its compounding effect on mortality rates.
We argue that robust surveillance mechanisms in conjunction with these types of studies will be indispensable to understand the true burden of bacterial infections.
We argue that robust surveillance mechanisms in conjunction with these types of studies will be indispensable to understand the true burden of bacterial infections.
Three syndromes are responsible for more than 75% of the estimated 7·7 million bacteria-related deaths that occurred in 2019.
Lower respiratory infections, bloodstream infections, and peritoneal and intra-abdominal infections would rank as the third, seventh, and 13th leading causes of death globally for 2019, respectively, all ahead of other causes such as HIV, colorectal cancer, or self-harm.
Lower respiratory infections have long been a global health priority, and bloodstream infections have arguably been included in the umbrella of more recent global sepsis initiatives;, however, intra-abdominal infections and peritonitis do not receive the same attention as other diseases with similar or lower fatal burden.
Although overlap exists in the management of peritoneal and intra-abdominal infections with other bacterial infections (eg, antibiotics and identification of the infection’s source), management of peritoneal and intra-abdominal infections poses unique challenges in that radiological imaging is often required to establish a source and surgical intervention might be needed to achieve source control.
Three syndromes are responsible for more than 75% of the estimated 7·7 million bacteria-related deaths that occurred in 2019.
Lower respiratory infections, bloodstream infections, and peritoneal and intra-abdominal infections would rank as the third, seventh, and 13th leading causes of death globally for 2019, respectively, all ahead of other causes such as HIV, colorectal cancer, or self-harm.
There is a substantial shortage of medical capacity and trained personnel in many LMICs to address peritoneal and intra-abdominal infections and other infections that require surgical intervention.
Recent estimates suggest 4·8 billion people do not have access to timely surgical services, with low-income countries estimated to have fewer than one provider per 100 000 population.
Compounding inadequate access to surgical services is the restricted availability of diagnostic radiology, with a recent analysis of ten LMICs across the Caribbean, South Asia, and sub-Saharan Africa finding that CT was available in only 6% of hospitals and ultrasound was available in only 50% of hospitals.
There is a substantial shortage of medical capacity and trained personnel in many LMICs to address peritoneal and intra-abdominal infections and other infections that require surgical intervention.
Recent estimates suggest 4·8 billion people do not have access to timely surgical services, with low-income countries estimated to have fewer than one provider per 100 000 population.
The remarkable geographical variation of responsible pathogens for a given infectious syndrome is highlighted by S aureus as the causative pathogen of bloodstream infection.
In the high-income super-region, S aureus caused 23% of deaths due to bloodstream infections that involved one of the 33 bacteria investigated, compared with only 5% of deaths due to bloodstream infections in the sub-Saharan Africa super-region.
This variation has profound implications on the empirical management of infections when a responsible pathogen has not yet been identified and breadth of coverage must be balanced against risk of antibiotic resistance.
The WHO essential medicines list provides global empirical antibiotic recommendations for various infectious syndromes; however, our findings suggest that a move towards region-specific empirical antibiotic recommendations might be more appropriate from an antibiotic stewardship and antimicrobial efficacy standpoint.
Region-specific guidance will also help in addressing inappropriate antibiotic use in LMICs, which is one of the key drivers of antimicrobial resistance.
We hope that these estimates might be used to guide empirical antibiotic use, yet data sparsity remains a major limitation in creating more granular estimates with sufficient confidence to inform individual clinicians in accordance with clinical needs and the aim to uphold antimicrobial stewardship.
The WHO essential medicines list provides global empirical antibiotic recommendations for various infectious syndromes; however, our findings suggest that a move towards region-specific empirical antibiotic recommendations might be more appropriate from an antibiotic stewardship and antimicrobial efficacy standpoint.
We should also acknowledge M tuberculosis, which was not included in our analysis.
One reason we did not do additional estimations for this important pathogen is because the global burden estimates provided by GBD 2019 and WHO are quite concordant and well established.,
A GBD study has shown that, in 2019, there were 9·65 million incident cases and 1·21 million deaths due to tuberculosis in both HIV-negative and HIV-positive individuals.
There was also a greater incidence and an excess burden in males, which is comparable with the burden of bacterial agents estimated in this study.
Geographically, most cases of tuberculosis in 2019 were found in the WHO regions of South-East Asia, Africa, and the Western Pacific,, which is comparable with the geographical spread of the burden of the 33 bacteria we investigated in this study.
Insufficient microbiological capacity has substantial effects on both population health estimates and the clinical care of individual patients.
Correspondingly, an urgent need exists to build microbiology laboratory networks and develop innovative surveillance strategies.
Identification of a responsible pathogen in sepsis and other severe infections can help inform optimal antibiotic choice and duration, and lead clinicians to probable sources of infection.
Without microbiological data, patients might remain on inappropriate or ineffective antibiotics that contribute to worse health outcomes and fuel the spread of antimicrobial resistance.
Practical antibiotic prescribing is also affected because the distribution of pathogens and local patterns of antimicrobial susceptibility are unknown, which hamper the development of dependable treatment protocols. In a recent study, investigators found that fewer than half of hospitals in ten LMICs had the capacity to do Gram staining, and we speculate that even fewer hospitals in this context could perform cultures and susceptibility testing.
Insufficient microbiological capacity has substantial effects on both population health estimates and the clinical care of individual patients.
Many locations have little or no microbiology data to inform local burden estimates, and so they must rely on modelled estimates to approximate the burden, resulting in wide uncertainty intervals.
The barriers to building microbiology capacity in LMICs have been well described, and overcoming these challenges requires greater investment and prioritisation of bacteriology capacity, and the development of national antimicrobial resistance surveillance networks.
Our study has several limitations, many of which are the result of data sparsity.
Input data for each modelling step has incomplete geographical coverage and is of varying quality for many LMICs, and we did not have data for 61 countries or territories for all three of our modelling steps. Hence, the locations where the burden is estimated to be the greatest are where the data are most scarce, which is an issue exacerbated by age-targeted surveillance protocols; this data scarcity should underscore the urgency of improving capacity and surveillance systems in LMICs. The identification of deaths in which infection had a role relied on International Classification of Diseases (ICD) coded deaths, which does not perfectly correlate with expert chart review. Our estimates of lower respiratory infections and urinary tract infections split infections into community-acquired versus hospital-acquired infections on the basis of whether ICD coding indicated the infection was an underlying or intermediate cause of death. However, this approach has not previously been validated and has the risk of misclassification. We assumed the same pathogen distribution among culture-negative as among culture-positive infections. This assumption could overestimate pathogens that are easier to detect and underestimate pathogens that are difficult to culture with the use of standard microbiological techniques (eg, culture-negative endocarditis might be caused by Bordetella spp or Coxiella spp, bacteria that are notoriously difficult to culture, although we expect the effect of this particular example on overall bacteria aetiologies to be quite small). We have a residual polybacterial category in which multiple possible causative pathogens were identified for a single infection; however, because many of these infections involved one or more of the 33 bacteria we investigated, this approach leads to an underestimation of the specified bacteria. Additionally, passive microbial surveillance data could have had some selection bias, particularly if cultures were not routinely drawn. In some locations, cultures might be drawn only if someone is critically ill or has not responded to treatment, which might overestimate more virulent or more resistant pathogens. Finally, this study is supported by the framework of and estimates from the GBD study, which has its own limitations that have been discussed elsewhere.
The 7·7 million deaths associated with the 33 pathogens we investigated are deaths that occurred in people with infections caused by one of these bacteria; …
… however, we cannot conclusively state that if all infections due to these 33 pathogens were eliminated, then 7·7 million deaths would have been prevented.
Many of these deaths were identified as deaths due to sepsis, when the underlying cause was non-infectious.
In a subset of these deaths, the underlying cause of death might have been so severe that a death would have occurred whether or not the infection took place.
For example, someone with terminal pancreatic cancer who dies from E coli peritonitis is counted the same as a neonate who dies of neonatal sepsis due to E coli.
However, most of the estimated 7·7 million deaths occurred when the infection with one of the 33 bacteria was the underlying cause of death, and in those cases, we could reasonably assume that those deaths would have been prevented if the infection had not occurred.
Placing infections into discrete categories of clinical syndromes opens up the discussion of how to address bloodstream infections, a syndrome that is not always distinct from other clinical syndromes and is often an intermediary between a precipitating infection and sepsis.
Our approach to infectious syndromes used a hierarchy process in which bloodstream infections were ranked the lowest — ie, if bloodstream infection was reported alongside any other infectious syndrome, the other infectious syndrome was used.
In other words, bloodstream infections as reported here were primary bloodstream infections for which the point of entry or other associated infectious syndromes could not be identified.
In Summary
In summary, our analyses show that bacterial infections are a clinically significant cause of health loss globally.
Five pathogens were each involved in more than 500 000 deaths in 2019: S aureus, E coli, S pneumoniae, K pneumoniae, and P aeruginosa.
Three infectious syndromes, each responsible for more than 1 million deaths in 2019, comprised more than 75% of deaths due to bacterial infections.
A sobering reality is that a high burden of treatable infections occurred in very young age groups.
Building stronger health systems with more robust diagnostic infrastructure, improved diagnostic imaging and microbiological capacity, and standardised workflows are crucial steps to address this substantial burden, together with implementing appropriate infection control and antimicrobial stewardship measures.
Essential prevention strategies include improved access to safe drinking water and sanitation facilities, increased rates of vaccination, new vaccine development, and improving access to the appropriate antibiotic for an infection.
There is a need to reconcile the right to antimicrobial access with non-judicious use, particularly with regard to expensive and newer generation antimicrobials.
Predictive mathematical modelling and further advancements in genomic epidemiology of infections will increase insights at the global level to understand pathogens’ evolution, epidemiology, and pathogenesis, and will better inform future approaches.
Originally published at https://www.thelancet.com.
Authors (top names in the list)
GBD 2019 Antimicrobial Resistance Collaborators
Correspondence to: Prof Mohsen Naghavi, Department of Health Metrics Sciences, Institute for Health Metrics and Evaluation, School of Medicine, University of Washington, Seattle,
Kevin S Ikuta, Lucien R Swetschinski, Gisela Robles Aguilar, Fablina Sharara, Tomislav Mestrovic, Authia P Gray, Nicole Davis Weaver, Eve E Wool, Chieh Han, Anna Gershberg Hayoon, Amirali Aali, Semagn Mekonnen Abate, Mohsen Abbasi-Kangevari, Zeinab Abbasi-Kangevari, Sherief Abd-Elsalam, Getachew Abebe, Aidin Abedi, Amir Parsa Abhari, Hassan Abidi, Richard Gyan Aboagye, Abdorrahim Absalan, Hiwa Abubaker Ali, Juan Manuel Acuna, Tigist Demssew Adane, Isaac Yeboah Addo, Oyelola A Adegboye, Mohammad Adnan, et al
References
See the original publication