NEJM Catalyst
Jason Arora, MD, MPH, MA, Jessica L. Mega, MD, MPH, Amy Abernethy, MD, PhD, and William Stadtlander, MBA, MSc
May 10, 2022
umich
Executive Summary
by Joaquim Cardoso MSc
Digital, Health and Care
Research Institute for Better Health for All
June 9, 2022
What is the need?
- Early in the Covid-19 pandemic, challenges emerged that pointed to the need for connecting diagnostic, clinical, and demographic data at scale to support real-time monitoring and decision-making by public health agencies.
- Driven by this urgent need, a number of public and private entities worked to deploy data programs across the United States to address the information fragmentation and to improve the public health response to Covid-19.
- Connected systems that continue to incorporate dynamic information are vital to pandemic response efforts, both today and in future public health efforts.
An overview of the article
- In this article, leaders from Verily Life Sciences share their experiences in building a Covid-19 Research platform (C19R) to support a cross-country program for Covid-19 testing that demonstrates the potential for linking community-based diagnostic, clinical, demographic, and other data to support public health decision-making at scale.
- The program also creates the infrastructure for a learning system where public health actions can be informed by aggregated clinical care data, evolving insights, and ongoing evidence generation, thereby linking research and care delivery.
Outline of the publication
- Introduction
- Software Platform
- Processing and Returning Test Results in Real Time
- Ensuring Robust Data Collection
- Connecting Diagnostic, Clinical, and Demographic Data to Understand the Covid-19 Pandemic Better
- Covid-19 and Related Research Opportunities
- An Integrated Data Infrastructure Can Serve as an Early-Warning System for Population Health Management
What is the message of the paper?
An Integrated Data Infrastructure Can Serve as an Early-Warning System for Population Health Management
- In a pandemic, there is a need for robust, real-time, and — crucially — connected community data from multiple sources to inform a timely and equitable public health response.
- The program described herein demonstrates the value, broadly, of capturing and using real-world data from community testing and, specifically, the power of linking Covid-19 vaccine, diagnostic testing, and local public health management data.
- It represents a practical infrastructure that allows for the interrelationship of individual-level information (e.g., vaccination status with PCR test positivity), which can be aggregated at the population level (e.g., by county) to support public health management, as well as the ability to close the loop and address critical research questions.
It is a working example that could tie together currently fragmented elements into a learning health and clinical care system.
ORIGINAL PUBLICATION (full version)

Connecting Real-World Data to Support Public Health Efforts
NEJM Catalyst
Jason Arora, MD, MPH, MA, Jessica L. Mega, MD, MPH, Amy Abernethy, MD, PhD, and William Stadtlander, MBA, MSc
May 10, 2022
Introduction
When the Covid-19 outbreak was declared a pandemic by the World Health Organization (WHO) in early 2020, the need for a system for obtaining and returning laboratory results (e.g., SARS-CoV2 PCR tests, aka PCR) to inform clinical and public health decision-making in real time became clear; early on, the existing options were distributed and labor-intensive.
Numerous entities quickly moved to stand up services to triage diagnostic tests to people at the greatest risk and to track Covid-19 cases.,
As a health technology company, Verily (an independent subsidiary of Alphabet Inc.) began working with public health departments at the local, state, and national levels in the United States to develop a digital, end-to-end screening and testing platform within days of the WHO announcement. Public health departments also championed efforts to allow people to opt into research programs that would inform the understanding of the natural history of the infection and accelerate the development of treatments for Covid-19.
From our prior experience with the Project Baseline Health Study (PBHS), we leveraged our platform, which was designed to support scalable research and its integration into practice, to develop the Covid-19 testing platform.
Supported by more than 1,000 Google and Verily employees who volunteered additional personal time to work on the project, mostly on evenings and weekends, and several invaluable grassroots partners across California (including local police and fire departments), we began working on three foundational elements of the program:
testing sites, adapting our scalable software platform to allow for longitudinal Covid-19 data collection, and enabling the connecting of the data as soon as it was collected.
This allowed public health and clinical stakeholders to use the data to inform policy decisions and clinical actions, such as connecting people who test positive to appropriate therapeutics.
For example, Verily’s Covid-19 Research platform (C19R) was used to recruit participants for different clinical studies including a Covid-19 antibody study, Covid-19 vaccine phase III study, Covid-19 vaccine long-term safety and surveillance study, and Covid-19 foundational research study.
We learned that it was possible for a single software layer to plug into and connect multiple parties on a large scale, to support public health planning — in this case: pharmacy testing sites, laboratories, local public health departments, users of the testing service, and clinicians.
We learned that it was possible for a single software layer to plug into and connect multiple parties on a large scale, to support public health planning — in this case: pharmacy testing sites, laboratories, local public health departments, users of the testing service, and clinicians.
Software Platform
In parallel, we rapidly evolved Verily’s PBHS software platform to support this new national PCR testing program.
A goal was to simultaneously support clinical management of Covid-19, public health monitoring, and new research study opportunities in the context of the pandemic. The platform already had features for securely and privately managing the health data of study participants in a research setting, and we built additional functionality to meet regulatory requirements and other standards for use in clinical care. The adapted software platform is scalable and flexible to register and dynamically screen users of the service for testing eligibility, enable user consent, schedule testing appointments, and return testing results to testing service users and laboratories. We also leveraged the platform to allow testing service users to opt-in to research and to conduct study activities in support of evidence generation.
We learned that it was possible for a single software layer to plug into and connect multiple parties on a large scale, to support public health planning — in this case: pharmacy testing sites, laboratories, local public health departments, users of the testing service, and clinicians.
This occurred all in the context of a changing public health environment in which everyone was rapidly learning about the clinical characteristics and epidemiology of a new disease, and in which national and local public health authorities were implementing heterogeneous policies.
Another limitation of the effort is that we do not have definitive examples of how the data was used, but we do know that public health authorities requested different data and that it was used.
Such an effort is not without challenges, of course.
When we added a new processing lab, for example, we needed to make sure that the lab requisitions and data were integrated so that we accurately tracked patients and their samples in the system. This process could take weeks to implement. Another limitation of the effort is that we do not have definitive examples of how the data was used, but we do know that public health authorities requested different data and that it was used.
Processing and Returning Test Results in Real Time
The process and data flows were built for ease of use by all parties involved — including the testing service user, the Rite Aid testing site, the physician network, lab processor, and local department of health.
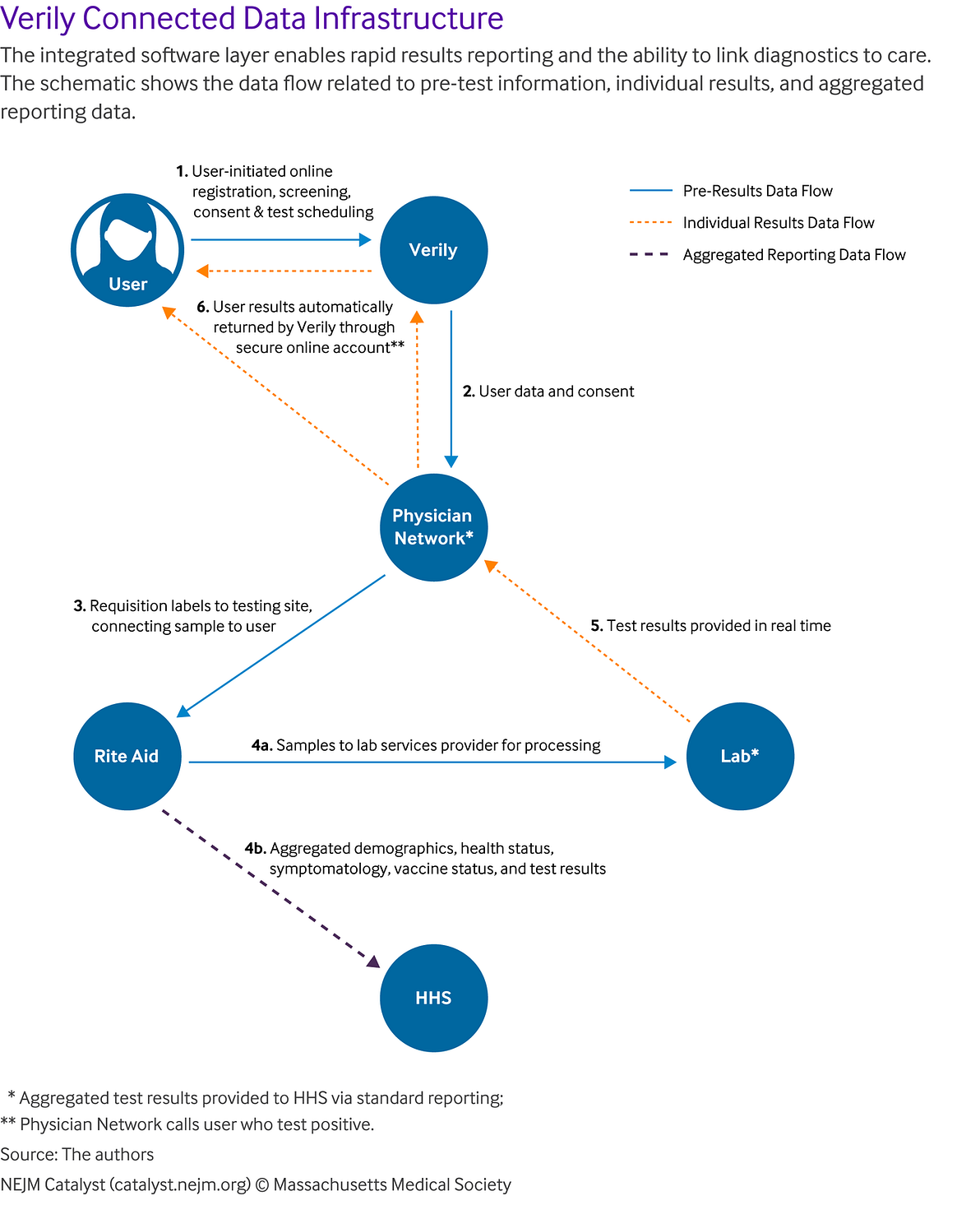
In particular, all users’ consent and registration data were collected online at the time the user registered and scheduled their test. This reduced the administrative burden of data entry at the testing site, with only an ID check and sample being collected. All data was integrated between the different parties so that when a user scheduled a test, it triggered the physician network to order a requisition, which provided requisition labels to the testing site, allowing the collected sample to be connected to the user. Once the lab completed processing of the sample, the results were automatically returned to the user through their secure online account. For positive cases, the physician network was notified to call the user to answer questions. The data, including test results and other demographic and vaccine status data, were continuously provided to HSS. This integration and process enabled rapid results-reporting and the ability to link diagnostics to care ().
Ensuring Robust Data Collection
In expanding the software platform and a connected data infrastructure, the team had to navigate several key areas:
First, the permissions needed to ensure people were able to consent to multiple uses of their data.
To address this, within the single platform, we developed separate consent procedures for clinical care and public health uses, as required by federal and state departments of health, and a separate optional consent form for those interested in also being part of the research component. Users also receive results back (return of results) on an individual level and/or aggregate level (de-identified), depending on the research initiative they sign up for, to provide value back to the user.
Second, to support better and more equitable clinical care, we needed to make the testing service easy to use and accessible to as many people as possible.
Because HHS provided the funding, the user was able to get tested at no cost. The user portal is available on the Web and mobile, in English and Spanish. In early 2021, consent was expanded from those 18 and over only, to include participants aged 4 to 17 with parental consent. Consequently, we were able to develop a sizable, robust, and diverse data set. As of March 2022, the Covid-19 testing data set included approximately 4 million unique users of the testing service who have had a combined total of more than 6 million tests from the Rite Aid locations.
Finally, we had to ensure that all these interconnected systems met all federal and state reporting requirements, including multiple adjustments in reaction to the rapidly evolving pandemic.
As of March 2022, the Covid-19 testing data set included approximately 4 million unique users of the testing service who have had a combined total of more than 6 million tests from the Rite Aid locations.
Connecting Diagnostic, Clinical, and Demographic Data to Understand the Covid-19 Pandemic Better
A key feature of the program is that it allows for the interrelationship of community-based diagnostic data (e.g., PCR test results), clinical data (e.g., vaccine status), and demographic data on a national scale.
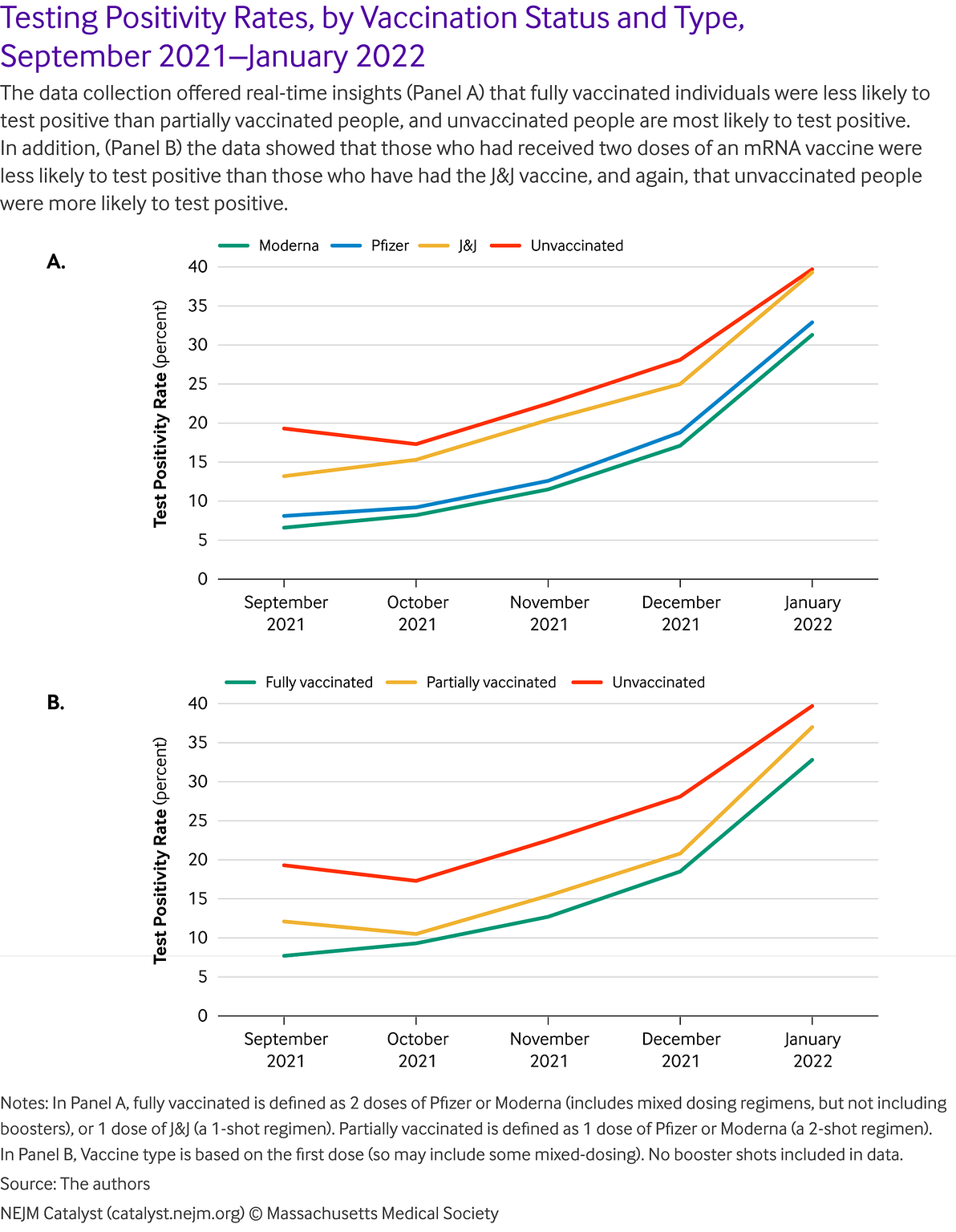
The ability to link vaccination data with testing data within the same data ecosystem, for example, has meant that the program has been able to observe test positivity rate over time, segmented by vaccination type. Population-level, aggregate, non-identifiable analyses of connected testing, vaccine, and demographic data show trends in line with expected findings and prospective cohort clinical trial findings.
For example, in a sample of more than 1 million testing service users between September and December 2021, we observed that the likelihood of testing PCR positive correlates with expected trends by vaccination status and type. Fully vaccinated people are less likely to test positive than partially vaccinated people, who are less likely to test positive than unvaccinated people. Those administered with two doses of an mRNA vaccine are less likely to test positive than those who have had the Johnson & Johnson vaccine, who are less likely to test positive than unvaccinated people. These results have many potential confounders (e.g., people who got vaccinated are potentially more likely to get tested) but the overall trend aligns with expected patterns ().
There are a number of ways to potentially leverage this approach to support public health goals and efforts.
In New York, for example, the NY State Back-to-School program was able to draw on the platform data by working with HHS, which oversaw the actioning of the data, to develop an easy way to capture school district information for participants under 19 years old.
This data was shared with the state so they could monitor how many Covid-19 cases a school district had in the week prior to school starting.
This would allow a school district to take appropriate action, e.g., to recommend students get tested, to implement social behaviors, or to close schools.
The platform creates the opportunity for such action through the generation of relevant data and the connection of different data sources for new insights.
Because public health officials are able to link testing and vaccination data, they can monitor for patterns of breakthrough infections in communities.
They are also able to understand lead time for breakthrough cases (i.e., days since last vaccination to a positive test result) by vaccination status and type. They can monitor for hot spots and trends.
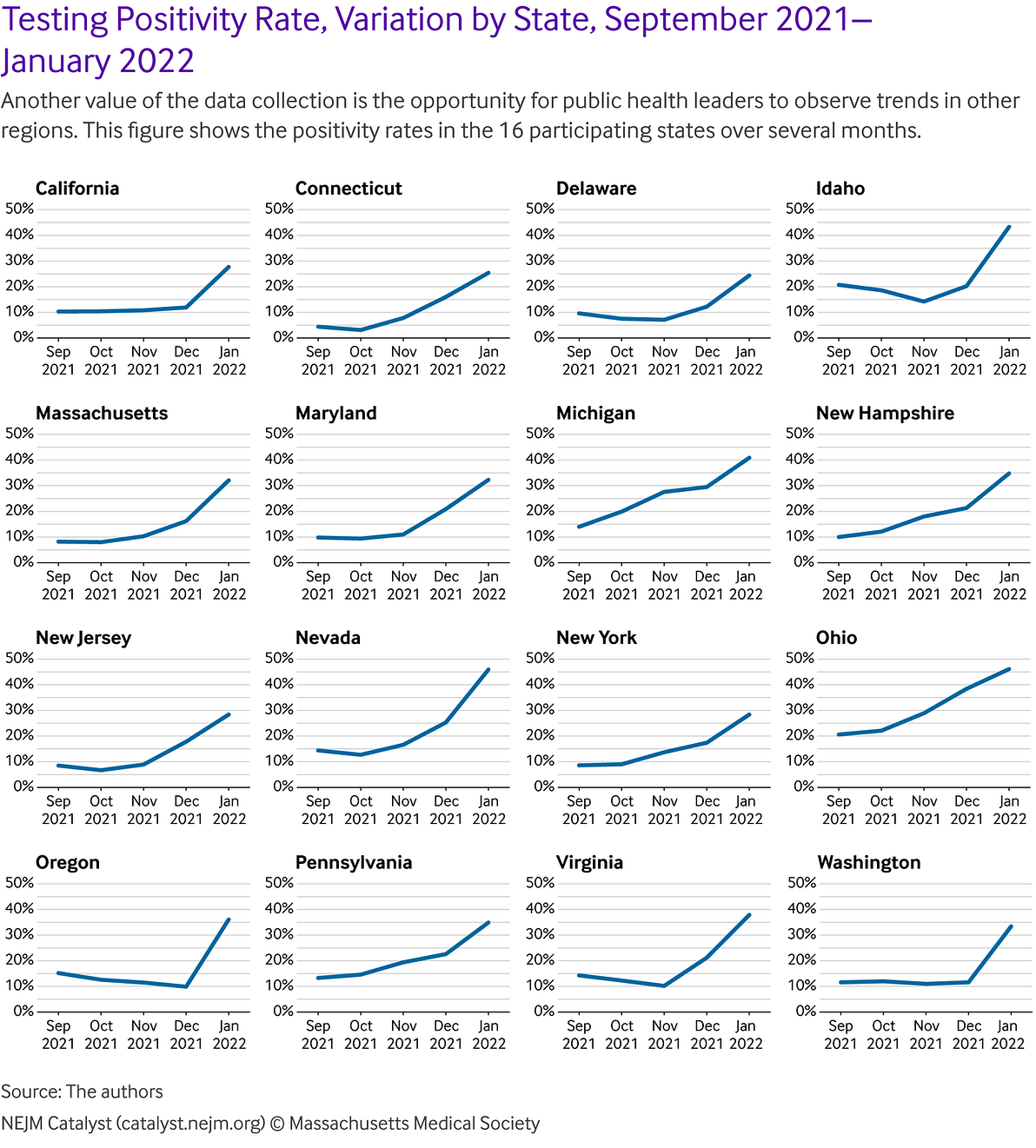
For example, test positivity increased in several localities over November and December 2021, and then began to reduce again in January 2022. This was likely due to the emergence of the Omicron variant ().
There are a number of ways to potentially leverage this approach to support public health goals and efforts.
e.g.Because public health officials are able to link testing and vaccination data, they can monitor for patterns of breakthrough infections in communities.
The program can, indeed, systematically collect variant data, which, when viewed with the database of demographic and user-specific longitudinal data can be incorporated with expanded individual medical histories to help contextualize such findings, providing insights with even greater resolution and generating opportunities to connect users to more tailored clinical care.
For example, the connected data infrastructure of the Verily platform could help identify risk factors and high-risk groups in near real-time, which can inform public health policy and protective actions for those groups.
And, if linked with the individual’s medical record or history, this could trigger an alert to the patient’s physician.
This also applies at the population level, where a high-risk patient cohort could be identified in a similar manner.
The ability to link vaccination data with testing data within the same data ecosystem, for example, has meant that the program has been able to observe test positivity rate over time, segmented by vaccination type.
The ability to link vaccination data with testing data within the same data ecosystem, for example, has meant that the program has been able to observe test positivity rate over time, segmented by vaccination type.
Covid-19 and Related Research Opportunities
Users of the Verily Covid-19 testing program are invited to participate in ongoing Covid-19 research opportunities to support preparation for the next wave of the pandemic and address related public health concerns and misconceptions.
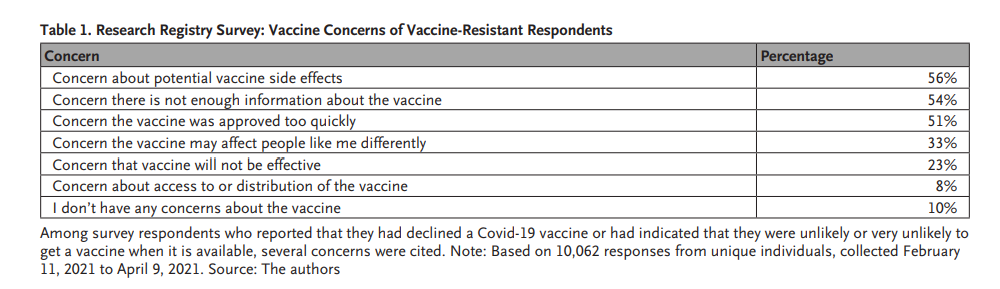
To enable this learning, we developed a Covid-19 Research Registry, which has provided the opportunity to generate richer, longitudinal real-world data on exposure, social distancing and mask wearing habits, mental health and well-being, timely topics such as one’s gathering plans over an upcoming holiday period, and even vaccine perceptions. As of March 2022, nearly 240,000 participants have opted into the research registry. From that pool, several thousand have responded to survey questions; for example, among the 1,006 respondents who have declined a Covid-19 vaccine or not been offered the vaccine, the top concerns cited were potential side effects (56%), lack of information about the vaccine (54%), and a belief that the vaccine approval process was too fast (51%) ( Table 1).
Respondents most often cited isolation as the as a stressor experienced within the preceding 2 weeks, although the response rate for that choice remained at less than 30% over a 6-month period ( Table 2).
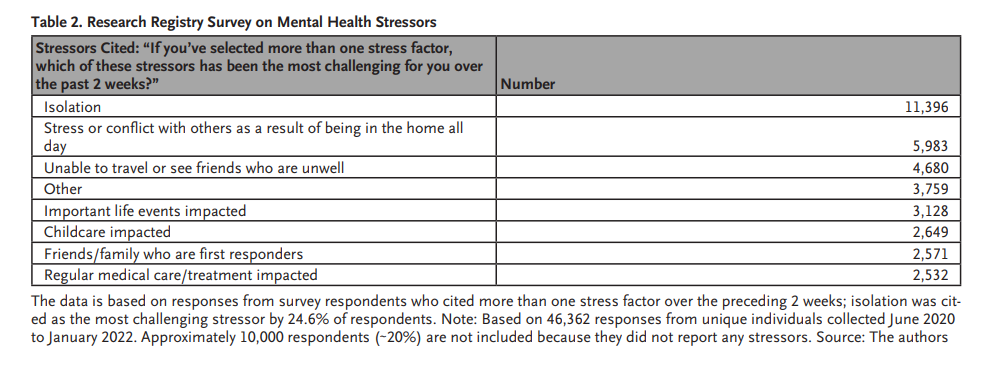
Stressors Cited: “If you’ve selected more than one stress factor, which of these stressors has been the most challenging for you over the past 2 weeks?” Number
The ability to connect this Covid-19 Research Registry survey data set back to clinical, laboratory, and demographic data means that further insights about the pandemic can be derived, which can inform policy at the federal, state, and local levels.
This approach can be taken for other data sets, too, such as those for vaccine pharmacovigilance and tracking of adverse events.
Indeed, testing service users have the choice to engage in Covid-19 therapeutic and vaccine studies, and to enroll in other non-Covid-19 studies, which highlights another potential benefit of this type of multi-sourced integrated system.
An Integrated Data Infrastructure Can Serve as an Early-Warning System for Population Health Management
In a pandemic, there is a need for robust, real-time, and — crucially — connected community data from multiple sources to inform a timely and equitable public health response.
The program described herein demonstrates the value, broadly, of capturing and using real-world data from community testing and, specifically, the power of linking Covid-19 vaccine, diagnostic testing, and local public health management data.
It represents a practical infrastructure that allows for the interrelationship of individual-level information (e.g., vaccination status with PCR test positivity), which can be aggregated at the population level (e.g., by county) to support public health management, as well as the ability to close the loop and address critical research questions.
It is a working example that could tie together currently fragmented elements into a learning health and clinical care system.
About the authors
Jason Arora, MD, MPH, MA
Head of Clinical Science Innovation, Clinical Studies Platforms and Head of Medical Affairs,
Verily Life Sciences LLC, South San Francisco, California, USA
Jessica L. Mega, MD, MPH
Co-Founder and Chief Medical and Scientific Officer, Verily Life Sciences LLC, South San Francisco, California, USA
Amy Abernethy, MD, PhD
President, Clinical Studies Platform, Verily Life Sciences LLC, South San Francisco, California, USA
William Stadtlander, MBA, MSc
Head of Global Life Sciences Marketing, Verily Life Sciences LLC, South San Francisco, California, USA
References
see original publication
Originally published at https://catalyst.nejm.org on May 10, 2022.