NEJM
Christine M. Lovly, M.D., Ph.D.
May 25, 2022
N Engl J Med 2022;
Lung cancer remains the leading cause of cancer-related death worldwide.1
Even in the context of early-stage disease, survival among patients with lung cancer lags behind that among patients with other common cancers, such as colon, breast, and prostate cancer.
The likelihood of 5-year survival remains modest,2 with patients having both local or regional relapse and metastatic relapse.
Although surgery with curative intent remains the foundation for management of early-stage lung cancer, these data speak to the urgent need for improvements in systemic control.
Lung cancer has seen tremendous advances over the past decade
With the advent of targeted therapies directed against mutant oncoproteins and immune-checkpoint inhibitors to promote host antitumor response, lung cancer has seen tremendous advances over the past decade, albeit predominantly in the context of metastatic disease.
In contrast, cytotoxic chemotherapy has remained the mainstay of treatment in the adjuvant (postoperative) context, with cisplatin-based regimens showing a 5.8-percentage-point improvement in disease-free survival and a 5.4-percentage-point improvement in overall survival at 5 years.3
Neoadjuvant (preoperative) chemotherapy approaches have resulted in similarly modest benefits4 and have been hindered by variability in implementation across different medical practices.
Recently, a surge of studies has been bringing new therapies into postoperative care.
In 2020, the Food and Drug Administration (FDA) granted approval to the first targeted therapy, osimertinib, for adjuvant use.
Less than a year later, atezolizumab became the first immune-checkpoint inhibitor to be FDA-approved for adjuvant use, heralding the dawn for much-needed advances in early-stage lung cancer.
Yet, questions remain regarding the magnitude of benefit associated with adjuvant therapy and how to best tailor such therapies according to disease stage, biomarker expression, and other patient-specific factors.
On the other side of the operative coin, the expansion of new therapies into the neoadjuvant context offers several possible advantages, …
… including reduced tumor burden before surgery (“downstaging”), the possibility of surgery with fewer complications, and the ability to assess therapeutic response on surgical resection of the tumor (“pathological response”).
Immunotherapy before surgery may also offer the advantage of enhanced T-cell priming and increased expansion of antitumor T cells, allowing continued immunosurveillance of micrometastatic disease after resection.5,6
Indeed, a series of promising early-phase trials of immune-checkpoint inhibitors for neoadjuvant use 7 paved the way for the international, randomized, phase 3 CheckMate 816 trial now reported by Forde et al. in the Journal. 8
In the trial, patients with stage IB to IIIA non–small-cell lung cancer were randomly assigned to receive three cycles of neoadjuvant nivolumab (an anti–programmed death 1 antibody) with platinum-doublet chemotherapy or chemotherapy alone before definitive resection.
The primary end points were event-free survival and pathological complete response.
The median event-free survival was 31.6 months in the nivolumab-plus-chemotherapy group and 20.8 months in the chemotherapy-alone group (hazard ratio for disease progression, disease recurrence, or death, 0.63).
Remarkably, a pathological complete response was detected in 24.0% of the patients in the nivolumab-plus-chemotherapy group, as compared with 2.2% in the chemotherapy-alone group (odds ratio, 13.94).
Benefits were observed across all the analyzed subgroups. Minimally invasive surgery was more common and pneumonectomy less common with nivolumab plus chemotherapy than with chemotherapy alone, with no delays in surgery observed and no substantial differences in treatment-related adverse events.
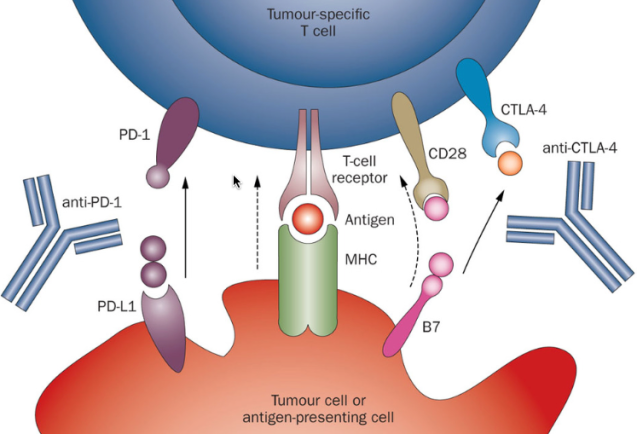
Results from this trial are expected to be practice changing, as evidenced by the FDA approval of neoadjuvant nivolumab plus chemotherapy on March 4.
However, several issues remain to be addressed:
First, is a pathological complete response predictive of event-free survival? Can event-free survival be used as a surrogate end point for overall survival?
Second, although not mandated for this trial, approximately 20% of the patients received postoperative therapy. Is adjuvant therapy necessary? What criteria should be used to select patients to receive adjuvant therapy?
Third, patients with tumors that harbored EGFR or ALK mutations were excluded from the trial.
Therefore, implementation of neoadjuvant therapies requires biomarker testing for patients with early-stage disease at the time of diagnosis, a considerable alteration in the routine practice of lung-cancer medicine.
To meet this requirement, protocols must be put in place to ensure successful tissue stewardship, avoiding the potential for delays in obtaining biomarker results needed to plan preoperative therapy.
Fourth, neoadjuvant therapy requires a disease-management team made up of experts in oncology, surgery, pathology, and more.
Implementation requires a multidisciplinary approach, which may be challenging in some centers.
How can these cutting-edge treatments be implemented broadly, regardless of location, to prevent widening the survival gaps that already exist for lung cancer,9 so that all patients may have access to potentially life-saving therapies?
Overall, although challenges remain, broad implementation of neoadjuvant therapy is expected to herald a new era for lung cancer
Overall, although challenges remain, broad implementation of neoadjuvant therapy is expected to herald a new era for lung cancer, coupling innovative treatments supported by a strong biologic rationale with timely assessment of antitumor responses in order to deliver on the promise of a decreased incidence of lung cancer–related death among patients with early-stage disease.
This editorial was published on April 11, 2022, at NEJM.org.
Author Affiliations
From the Department of Medicine, Division of Hematology and Oncology and Vanderbilt–Ingram Cancer Center,
Vanderbilt University Medical Center, Nashville.
Originally published at https://www.nejm.org.
Names mentioned
Nivolumab, Opdivo, Bristol