institute for continuous health transformation
(InHealth)
Joaquim Cardoso do Rosário
Founder, Chief Researcher & Editor
January 27, 2023
EXECUTIVE SUMMARY
There is a high potential of using MRI, specifically diffusion-weighted imaging (DWI), as a single modality for the detection of both osteoblastic and soft-tissue metastases.
- Whole-body DWI with a multicompartmental model is being explored as a simple solution for use of unenhanced MRI in metastasis detection rather than skeletal scintigraphy.
- However, there are limitations to the widespread adoption of whole-body MRI, such as lack of uniformity in performance and lack of standardized acquisition techniques.
The study by Conlin and colleagues aims to test the performance of a multicompartmental DWI model in patients undergoing whole-body MRI for prostate cancer staging, …
- … and sets the stage for use of multicompartmental DWI in discriminating metastases from normal marrow.
While there are limitations to the study, the results are promising …
- .. and the technique holds great potential for the investigation of various conditions …
- and for incorporation into local staging regimens to guide management in cancer patients
DEEP DIVE
Next-Generation Diffusion Imaging of Osseous Metastases
Radiology:Imaging Cancer(RSNA)
Daniel Margolis, MD
Jan 27 2023
Traditionally, radionuclide bone scans with technetium 99m–labeled diphosphonates have been the mainstay for detection and characterization of osteoblastic metastases.
While technetium 99m is widely available and examinations are relatively inexpensive and simple to interpret, they are sensitive for only osteoblastic metastases, requiring at least one other modality for the detection of soft-tissue metastases.
This has led to increasing interest in “next-generation imaging” including the use of exotic, often expensive imaging agents (1).
A single modality, especially one that does not require intravenous administration of contrast material or radiotracer, could potentially simplify patient assessment and reduce costs.
MRI may address this need with purportedly excellent soft-tissue and osseous marrow characterization, and use of fast techniques can obviate the need for intravenous contrast material administration (2,3).
In fact, one of the initial considerations for whole-body MRI was detection of osseous metastases (4).
However, these techniques often involved review of multiple pulse sequences in varied orientations, making this approach less efficient, and arguably less compelling, than a maximum intensity projection image from PET.
A comprehensive approach using diffusion-weighted imaging (DWI), which could produce maximum intensity projection images similar to those from PET, has been explored as a simple solution for use of unenhanced MRI in metastasis detection rather than skeletal scintigraphy (5).
Further, the METastasis Reporting and Data System provides comprehensive acquisition and reporting guidelines for whole-body DWI in advanced prostate cancer (6).
As promising as these techniques are, widespread adoption of whole-body MRI remains elusive.
One potential limitation is the lack of uniformity in performance of these techniques, with sensitivities ranging from 71% to 100% depending on the series (3,5).
This may, in part, stem from lack of standardized acquisition techniques.
The Radiological Society of North America Quantitative Imaging Biomarker Alliance has published a document with technical recommendations and methods of calibration for conventional DWI, but newer techniques may eliminate this requirement (7).

In this issue of Radiology: Imaging Cancer, Conlin and colleagues address this area of growing interest in oncologic MRI with their Technical Development article,
“A Multicompartmental Diffusion Model for Improved Assessment of Whole-Body Diffusion-weighted Imaging Data and Evaluation of Prostate Cancer Bone Metastases” (8).
Can MRI provide clinically useful performance for the detection of osseous metastases?
As with all novel imaging techniques, the first step after the technique is developed is to test its performance and refine it.
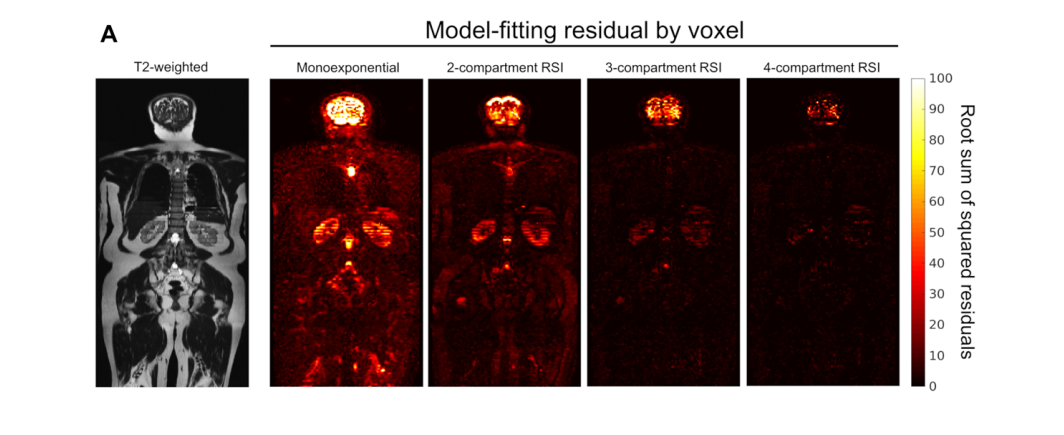
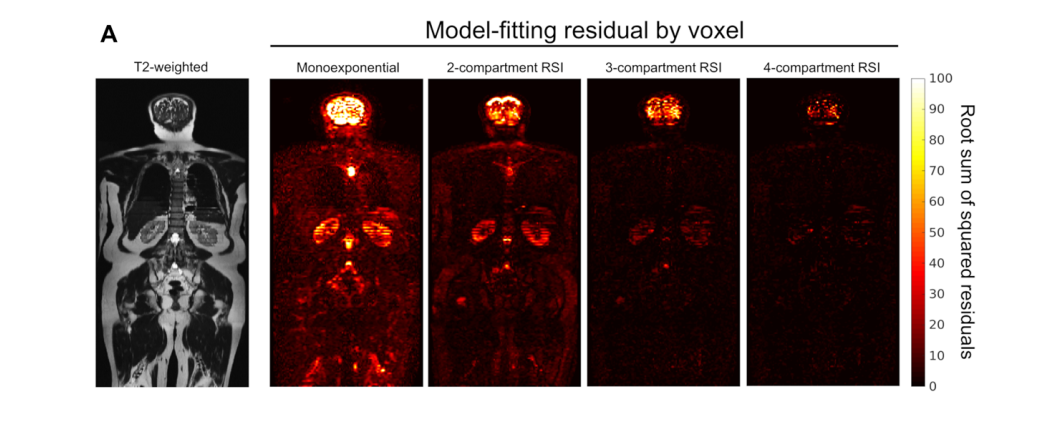
In the current study, the authors compare conventional DWI with a multicompartmental DWI model in patients undergoing whole-body MRI for prostate cancer staging.
The authors investigate the performance of various combinations of “compartments” based on a model derived from signal at multiple b values of a multishell diffusion acquisition with increasing numbers of directions by comparing the spread of Bayesian information criteria.
A similar technique has already shown excellent performance for primary prostate cancer (9).
The current investigation sets the stage for use of multicompartmental DWI in discriminating metastases from normal marrow.
This investigation is notable for its large number of initial participants, statistical rigor, and the number of potential models investigated.
The authors also provide a comprehensive yet nuanced review of the various compartments, which biologic aspects they likely reflect, and how these contribute to the accuracy of each model.
This discussion not only emphasizes the biologic basis for the observed results, showing that the experiment is not simply an empirical method to derive an MRI technique for identifying a tissue of interest, but also highlights possible use of this technique for other tissue and disease characterization, such as changes to the renal cortex.
As one might expect from such an early effort, there are some limitations.
Only 24 of the 139 participants recruited were found to have osseous disease, with 18 of these 24 having undergone prior systemic therapy, and histologic proof was missing for most if not all participants.
However, while such factors may limit the veracity and generalizability of the study results, generalizability was not the stated impetus for the investigation.
Additionally, the fact that most participants had received systemic therapy precludes discrimination between contributions of the neoplasia itself and its response to therapy to the models.
In the penultimate paragraph in the Discussion, the authors state, “we were unable to reliably compare the RSI [restriction spectrum imaging] signal properties of treated lesions to those of untreated lesions.”
This appears to purport that the model is broadly applicable, but the degree to which it is affected by treatment is unclear.
Regardless, the fact that the compartments discriminate diseased from uninvolved tissue is quite promising.

Such a rigorous and extensive investigation of the relative contribution of distinct diffusion compartments highlights the potential …
… to not only use this technique for the investigation of metastatic prostate cancer and myriad other conditions, but to refine the number of compartments analyzed, thus shortening the total scanner time.
A whole-body survey technique devoid of ionizing radiation and intravenous contrast material administration would be a great boon to the increasing number of patients surviving with cancer, prostate and otherwise.
The ability to incorporate this technique into local staging regimens holds great promise for a streamlined, comprehensive imaging study to guide management in these populations.
ORIGINAL PUBLICATION
A Multicompartmental Diffusion Model for Improved Assessment of Whole-Body Diffusion-weighted Imaging Data and Evaluation of Prostate Cancer Bone Metastases
Christopher C. Conlin, PhD • Christine H. Feng, MD • Leonardino A. Digma, BA • Ana E. Rodríguez-Soto, PhD • Joshua M. Kuperman, PhD • Rebecca Rakow-Penner, MD, PhD • David S. Karow, MD, PhD • Nathan S. White, PhD • Tyler M. Seibert, MD, PhD • Michael E. Hahn, MD, PhD* • Anders M. Dale, PhD*
From the Departments of Radiology (C.C.C., A.E.R.S., J.M.K., R.R.P., D.S.K., T.M.S., M.E.H., A.M.D.), Radiation Medicine and Applied Sciences (C.H.F., L.A.D., T.M.S.), and Neurosciences (A.M.D.), UC San Diego School of Medicine, 9452 Medical Center Dr, 4W 503, La Jolla, CA 92037; Human Longevity, Inc, San Diego, Calif (D.S.K.); CorTechs Labs, Inc, San Diego, Calif (N.S.W.); Department of Bioengineering, UC San Diego Jacobs School of Engineering, La Jolla, Calif (T.M.S.); and Halıcıoğlu Data Science Institute, UC San Diego, La Jolla, Calif (A.M.D.).