Global Forum Dia Global
Downloaded on April 16, 2022
Summary
Real-world data (RWD) and real-world evidence (RWE) are increasingly used to inform regulatory decisions around the world.
As a rapidly evolving regulatory topic, current discussions including regulators and industry highlight the need to clarify the regulatory standards for data quality, acceptable data sources, and analytical methods that support expanded use of RWE in assessing product efficacy/effectiveness and demonstrating its benefits, as well as evaluating product safety.
This article presents the results of a 2021 survey organized by industry associations in Brazil to assess the local regulatory environment and use of RWE/RWD studies in regulatory submissions to ANVISA, as well as the challenges and learnings from multiple companies.

In 2018, the US FDA published a framework to evaluate the potential application of RWE to support approving new indications for already licensed drugs or to support post-approval study requirements.
Since then, additional draft guidelines have emerged. According to the 2021 Aetion eBook The role of real-world evidence in FDA approvals, one of every two 2019 approved FDA submissions for new drugs and biologics included a real-world evidence study. In 2020, that figure jumped to 78%.
In the European context, RWE was used in 40% of marketing authorization applications and 18% of indication extensions that were submitted to EMA in 2018–2019 (Flynn et al., 2022).
In Japan, some orphan drugs have already been approved under the Act Securing Quality, Efficacy and Safety of Products based on the assessment of RWD.
Work to advance convergence around RWD policies and standards is progressing on a global basis through the International Council for Harmonisation, the International Coalition of Medicines Regulatory Authorities, and the Council for International Organizations of Medical Sciences.
The Brazilian Health Regulatory Agency (ANVISA) has not yet issued formal Guidance on the topic or issued official definitions for RWD and RWE.
However, regulatory discussions with internal decision-makers indicate that the Agency’s concept of these terms does not differ from what has been documented by other regulatory agencies. These perspectives can be seen in this article from 2020, authored by ANVISA.
Even without official definitions, RWD and RWE have routinely been included in documentation that ANVISA receives for evaluation.
However, structured information to characterize how they are used in making regulatory decisions in Brazil is limited. To address this gap, a pharmaceutical industry working group in Brazil developed a survey to assess industry’s perspective on the regulatory environment for RWD and RWE.
Two different surveys were sent to members of Sindusfarma (Pharmaceutical Products’ Industry Union) and ANAHP (National Association of Private Hospitals) in September and December 2021, designed to understand whether and how the industry was using RWD/RWE and how data source companies (clinical research organizations [CROs] and private hospitals) are collecting RWD.
Pharmaceutical Company Perspective
A total of 36 Sindusfarma members answered the survey, representing 44% of the Brazilian pharmaceutical market share, including national and multinational companies (Figure 1).
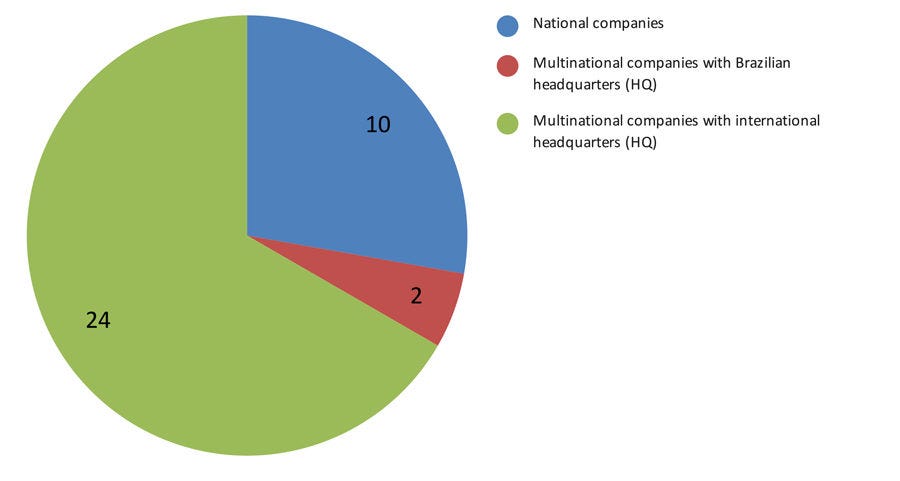
Figure 1: Type of pharma companies that responded to the survey
Twenty-five (70%) of these companies have already conducted or are conducting RWE studies with data from the Brazilian population, including national and multinational companies (Figure 2).
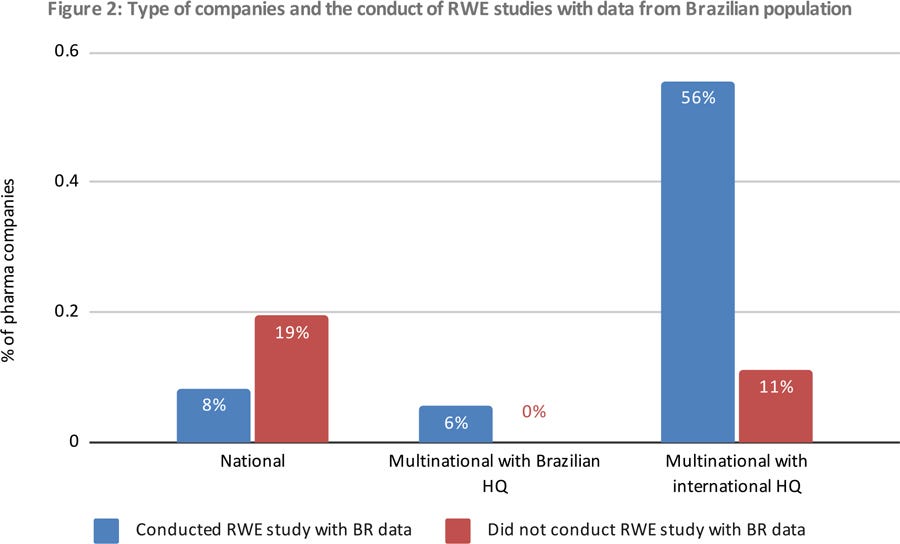
Figure 2: Type of companies and the conduct of RWE studies with data from Brazilian population
The survey collected additional information from these 25 companies about the types of data sources used, the therapeutic area, and the purpose of the RWE studies (Figures 3, 4, and 5). More than one choice was allowed for each answer, so percentages total more than 100%).
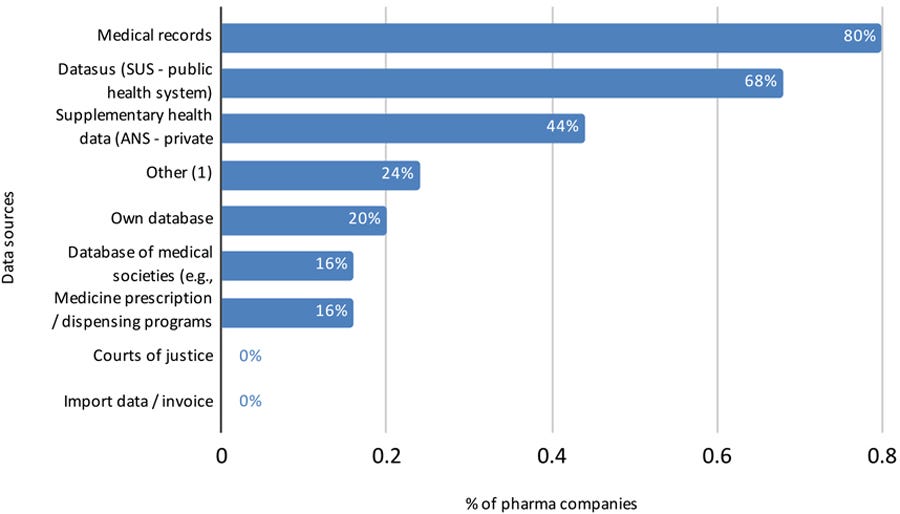
Figure 3: Data sources used by pharma companies to conduct RWE studies with data from Brazilian population
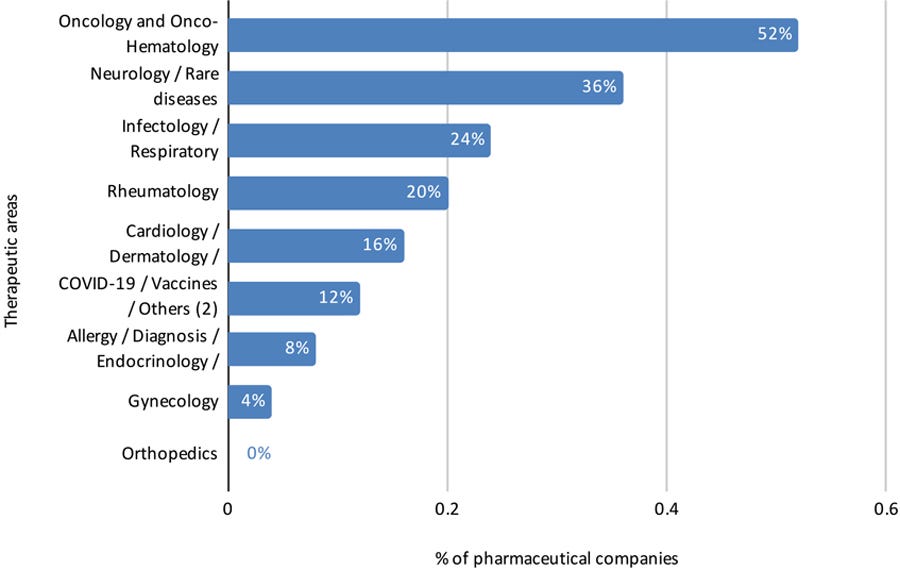
Figure 4: Therapeutic areas in which RWE studies with data from Brazilian population have been conducted by pharmaceutical industries
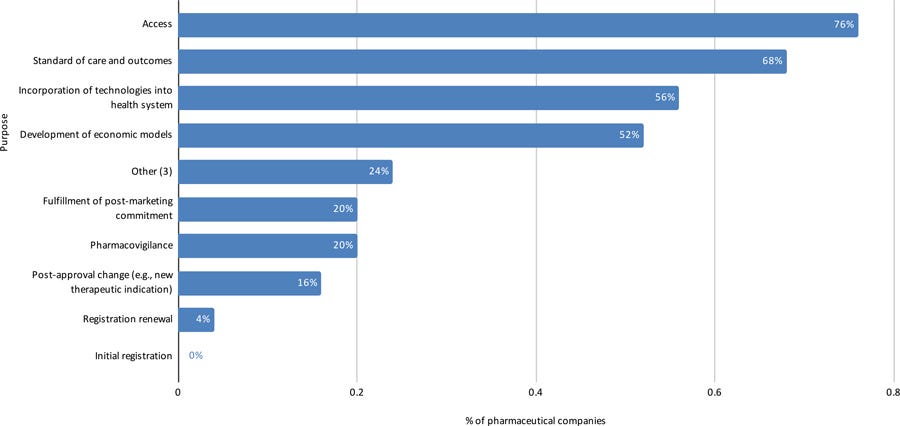
Figure 5: Purposes for which RWE studies with data from the Brazilian population have been conducted by pharmaceutical companies
From the total number of respondents (36), 16 (44%) have used RWE studies to support regulatory processes, requests, or other interactions with National Health Authorities.
In an open-ended question, these 16 respondents mentioned the authorities to which the RWE studies were sent (Figure 6).
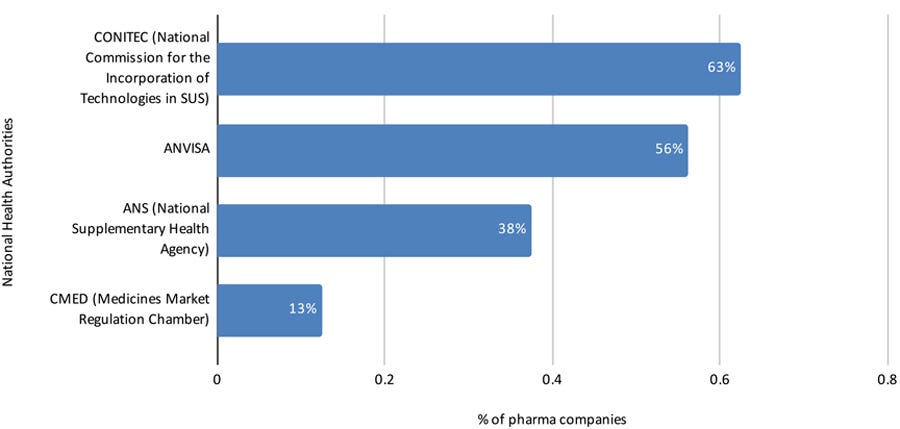
Figure 6: National Health Authorities to which RWE studies were sent for assessment
Thirty-eight percent of these 16 companies have conducted RWE studies in Brazil only, 31% in foreign countries only and 31% in both Brazil and foreign countries.
Nineteen respondents (53%) have submitted applications using RWD/RWE; Figure 7 displays the number of submitted applications.
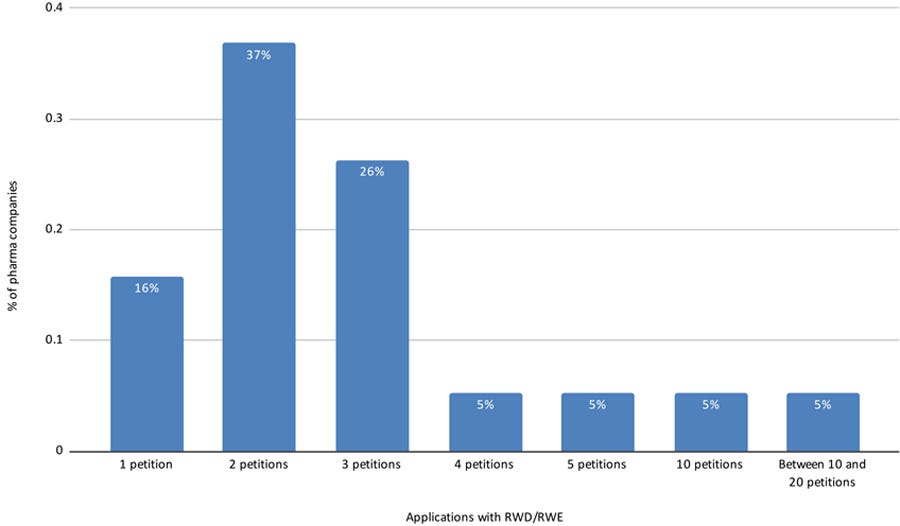
Figure 7: Number of applications with RWD/RWE submitted by pharmaceutical companies
Another open-ended question collected the main challenges to conducting RWE studies in Brazil, and the guidelines that the pharmaceutical industry uses to conduct them (Figures 8 and 9).
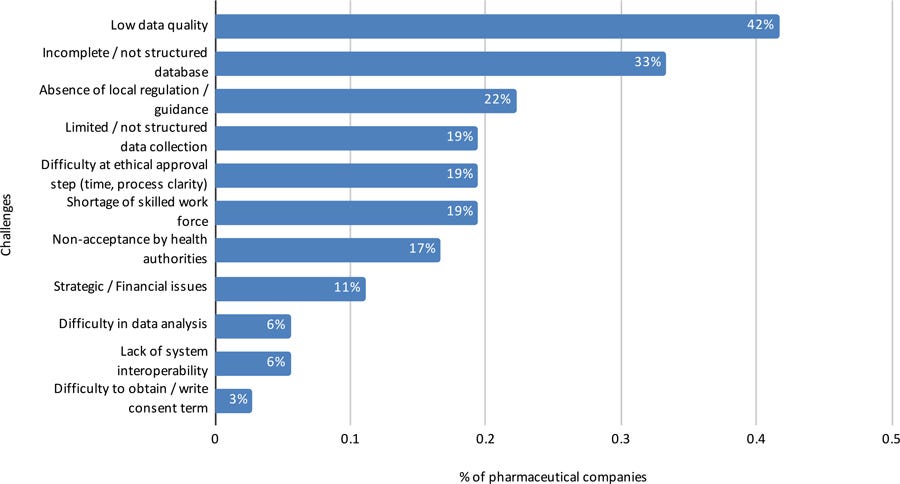
Figure 8: Challenges to conducting RWE studies in Brazil
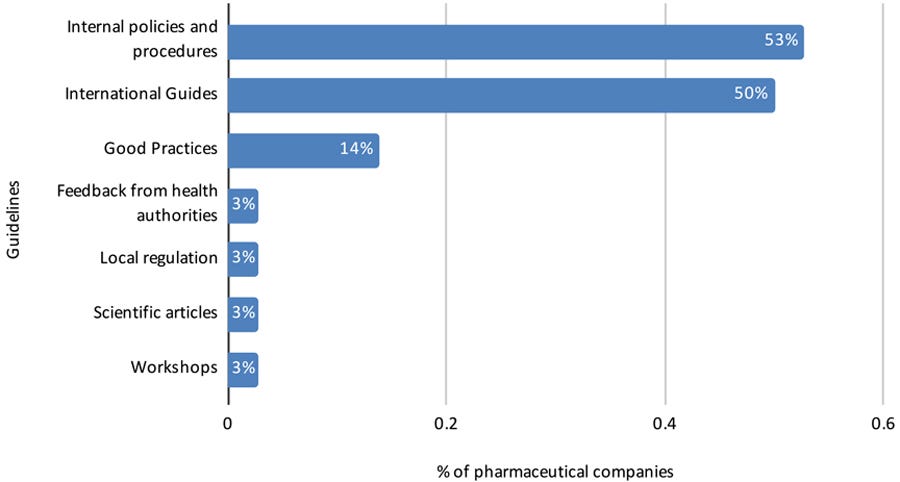
Figure 9: Guidelines used by pharmaceutical companies to conduct RWE studies
Seventeen (17) respondents (47%) reported that their company has a specific area or organizational structure for RWD/RWE in their company. But this area/structure is positioned in diverse locations: mainly located in the Medical Department but sometimes in Pharmacoeconomics, or in the Global Epidemiology structure, or in the specific disease franchise.
At some companies, this area is still being structured (“under construction”).
Perspective of Data Source Companies
Five data source companies-two private hospitals, two CROs, and one data analytics company-answered the survey.
All five collected or provided data for RWD/RWE purposes from different databases, and for several therapeutic areas and purposes (Figures 10, 11, and 12. More than one choice was allowed for each answer, so percentages total more than 100%).
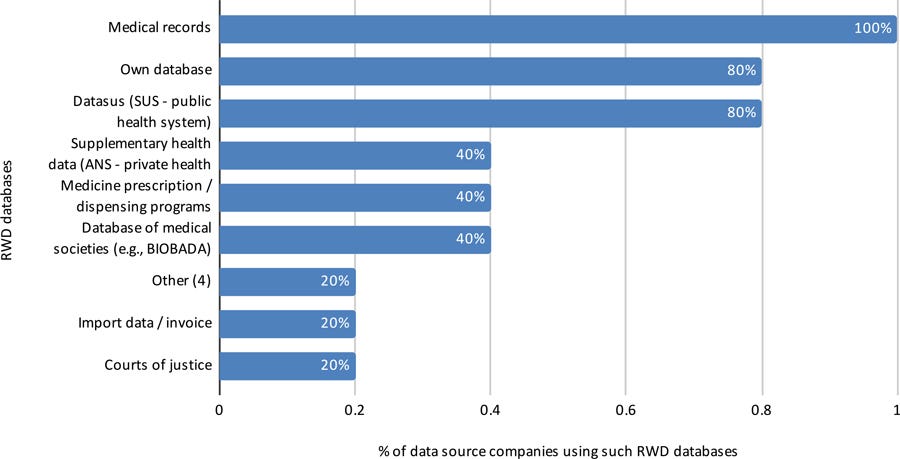
Figure 10: RWD databases used by data source companies in Brazil
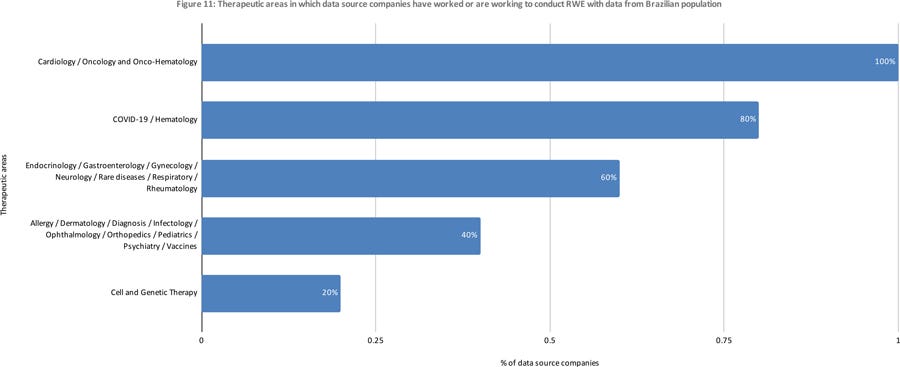
Figure 11: Therapeutic areas in which data source companies have worked or are working to conduct RWE with data from Brazilian population
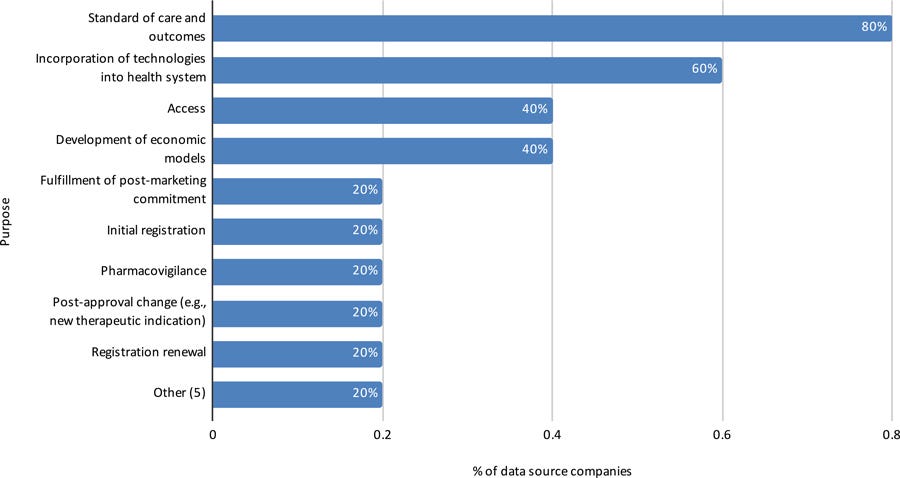
Figure 12: Purposes to which RWE with data from Brazilian population have been conducted
These respondents ensure patient data anonymity by adhering to Good Clinical Practices, using alphanumeric anonymization, their own procedure in compliance with the Brazilian General Data Protection Regulation, and artificial intelligence.
Four data source companies reported that data are standardized by terms and format; all five reported that data are organized/structured to evaluate specific outcomes for a population (Figure 13).
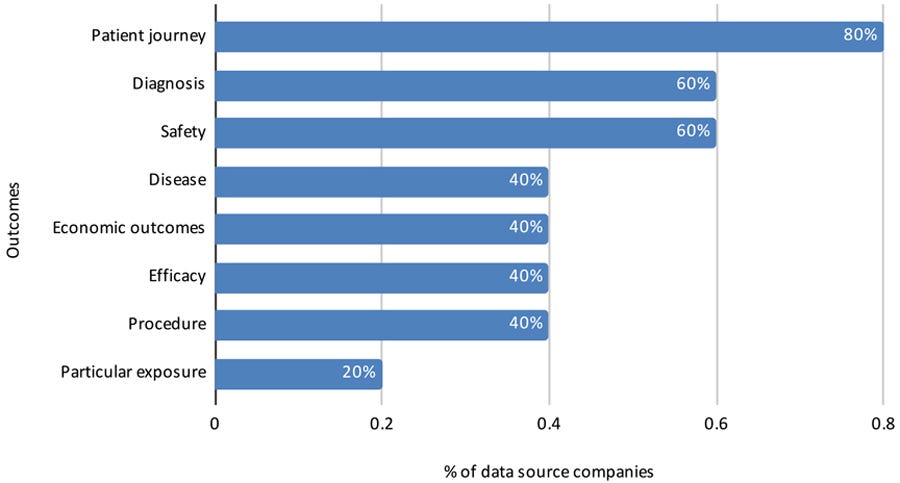
Figure 13: Outcomes evaluated
To ensure integrity and quality of the data collected from these sources, respondents apply standard operating procedures (SOPs) as part of their company’s quality systems, which use proprietary technology in partnership with a third-party company or their own artificial intelligence system.
They also encourage caution during data collection, analysis of data consistency, and data tracking.
In another open-ended question, respondents commented about the challenges of conducting RWE studies in Brazil (Figure 14).
Lessons learned by these data source companies, in addition to the value of applying SOPs, include potential use of hybrid studies; use of statistical methods to reduce bias; and the need for more robust technology and more dynamic technology platforms, such as applying artificial intelligence to unstructured text contained in clinical examination and medical records.
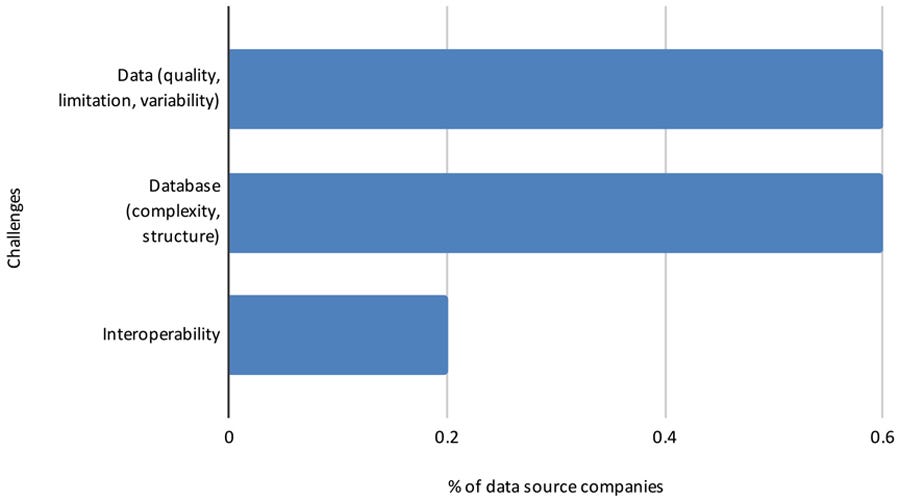
Figure 14: Challenges to conducting RWE studies in Brazil
All data source companies have ongoing RWE studies with hospitals and clinics.
Respondents further suggested that the best ways to expand these studies to other hospitals or clinics include agreements with groups of providers and data collection training.
Database structuring and formatting, data quality analysis, and data consistency analysis are also important.
No health authority has conducted an audit on any of the respondents’ datasets.
Insights, Global Context, and Conclusions
Survey results confirm the growing use of RWD/RWE by the pharmaceutical industry in Brazil.
Most respondents (76%) applied RWE to support drug access in Brazil, and 56% mentioned ANVISA as the health authority to which RWE studies were submitted.
This suggests that, even though RWE is widely used to support regulatory submissions at ANVISA, its use for regulatory decision-making remains a “work in progress” in Brazil:
Only five of the 25 companies which have used or are conducting RWE studies are Brazilian companies (or multinational companies with headquarters in Brazil).
… even though RWE is widely used to support regulatory submissions at ANVISA, its use for regulatory decision-making remains a “work in progress” in Brazil …
Extensive work and dialogue around RWD/RWE continue throughout our current global regulatory scenario, and there are multiple opportunities for multilateral collaboration among regulators and other stakeholders working toward convergence and common understanding in these regulatory approaches.
A certain level of global harmonization around RWE is key to ensuring better and more widespread use of globally generated data and avoiding duplicating work. ]
In this sense, the principles of regulatory reliance, as outlined by the World Health Organization (WHO) and supported by the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)-already a reality for international clinical trials-could be of use to ANVISA for RWE.
… the principles of regulatory reliance, as outlined by the World Health Organization (WHO) and supported by the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)-already a reality for international clinical trials-could be of use to ANVISA for RWE.
Although no formal conclusions can be derived because of the limited number of data source company participants in this survey, some trends can still be observed.
Every respondent indicated that the data collected are organized in a structured way that allows disease endpoints assessment, and that their organization applies data privacy and data protection practices.
Four of these five respondents reported that collected data are standardized.
This suggests that data source companies are increasingly aware of the need to comply with data privacy regulations while providing higher quality, curated data.
…data source companies are increasingly aware of the need to comply with data privacy regulations while providing higher quality, curated data.
Pharmaceutical and data source companies indicate that the most common challenges in using RWD/RWE include low data quality, incomplete databases, absence of local regulations/guidelines, a relatively unskilled work force, lack of systems interoperability, and non-acceptance by health authorities and ethics committees.
… the most common challenges in using RWD/RWE include: (1) low data quality, (2) incomplete databases, (3) absence of local regulations/guidelines, (4) a relatively unskilled work force, (5) lack of systems interoperability, and (6) non-acceptance by health authorities and ethics committees.
With these challenges in mind, potential solutions and opportunities to advance the use of RWE in Brazil include:
- Regulatory convergence to global guidelines and clear definition of what constitutes fit-for-purpose evidence in a particular regulatory context, to optimize research and development and accelerate patient access to innovative medicines.
- Greater transparency from ANVISA in detailing the use of RWE in public assessment reports and specifying why RWD/RWE is considered (or not considered) in regulatory decision making, to allow sponsors to learn from each other’s experiences.
- Foster knowledge sharing and building local capabilities among all stakeholders through workshops with local and global academic, regulatory, and industry representatives.
- Leverage local databases through the Brazilian National Digital Health Strategy or the ANAHP’s Clinical Endpoints Program to encourage collaboration with stakeholders (patients, payers, industry, government, regulatory agencies, and health professionals) and demonstrate the importance of high-quality data collection.
- Conduct an RWE pilot project between companies and agencies, allowing interaction and discussion on the use of RWD/RWE, and improving transparency and local data reliability.
… potential solutions and opportunities to advance the use of RWE in Brazil include several efforts (see details above)
Local policy incentives (such as guidance documents or frameworks) also need to be put in place to address the concerns of regulators and sponsors.
Disseminating best practices will further support the collection of comprehensive and high-quality RWD, as well as their assessment via robust scientific and analytical methods.
Finally, the subsequent review, endorsement, and communication of research results conducted using such data sources in a transparent manner will build trust and serve as an important enabler of their trustworthiness as evidence for use in regulatory decision-making.
References available upon request.
Authors
Camila Abreu, Elisangela Rorato, Ding Xu, Priscila Perrud, Débora Germano,
Renata Gonçalves, Letícia Moreira, Marcela Bronzeri, Graziella Matsumura, Cibele Boscolo, and Renata Corrêa
Professionals in pharmaceutical industries associated with Sindusfarma or Interfarma
Acknowledgments
The authors recognize Sindusfarma, Interfarma and ANAHP for their collaboration and support in the survey data collection.
Originally published at https://globalforum.diaglobal.org on March 29, 2022.