Is the second most frequent malignancy (after lung cancer) in men worldwide
Epidemiology of Prostate Cancer
World Journal of Oncology
Prashanth Rawla
Published online 2019 Apr 20. doi: 10.14740/wjon1191
medindia
Abstract
- Prostate cancer is the second most frequent cancer diagnosis made in men and the fifth leading cause of death worldwide.
- Prostate cancer may be asymptomatic at the early stage and often has an indolent course that may require only active surveillance.
- Based on GLOBOCAN 2018 estimates, 1,276,106 new cases of prostate cancer were reported worldwide in 2018, with higher prevalence in the developed countries.
- Differences in the incidence rates worldwide reflect differences in the use of diagnostic testing.
- Prostate cancer incidence and mortality rates are strongly related to the age with the highest incidence being seen in elderly men (> 65 years of age).
- African-American men have the highest incidence rates and more aggressive type of prostate cancer compared to White men.
- There is no evidence yet on how to prevent prostate cancer; however, it is possible to lower the risk by limiting high-fat foods, increasing the intake of vegetables and fruits and performing more exercise.
- Screening is highly recommended at age 45 for men with familial history and African-American men.
- Up-to-date statistics on prostate cancer occurrence and outcomes along with a better understanding of the etiology and causative risk factors are essential for the primary prevention of this disease.
Keywords: Prostate cancer, Epidemiology, Incidence, Mortality, Trends, Survival, Etiology, Risk factors, Prevention
FULL VERSION

Introduction
Prostate cancer is the second most frequent malignancy (after lung cancer) in men worldwide, counting 1,276,106 new cases and causing 358,989 deaths (3.8% of all deaths caused by cancer in men) in 2018 [ 1, 2].
The incidence and mortality of prostate cancer worldwide correlate with increasing age with the average age at the time of diagnosis being 66 years. Of note, for African-American men, the incidence rates are higher when compared to the White men, with 158.3 new cases diagnosed per 100,000 men and their mortality is approximately twice as White men [ 3].
Reasons for this disparity have been hypothesized to differences in social, environmental and genetic factors.
Although 2,293,818 new cases are estimated until 2040, a small variation in mortality will be observed (an increase of 1.05%) [ 4].
Prostate cancer may be asymptomatic at the early stage and often has an indolent course, and may require minimal or even no treatment.
However, the most frequent complaint is difficulty with urination, increased frequency, and nocturia, all symptoms that may also arise from prostatic hypertrophy.
More advanced stage of the disease may present with urinary retention and back pain, as axis skeleton is the most common site of bony metastatic disease.
Many prostate cancers are detected on the basis of elevated plasmatic levels of prostate-specific antigen (PSA > 4 ng/mL), a glycoprotein normally expressed by prostate tissue.
However, because men without cancer have also been found with elevated PSA, a tissue biopsy is the standard of care to confirm cancer’s presence.
Diet and physical activity play an important role in prostate cancer development and progression.
Dietary factors are mainly associated with the observed worldwide and ethnic differences in the incidence rates of prostate cancer [ 5– 9].
Most studies are devoted not only into identifying genes involved in the inherited form of prostate cancer but also the mutations occurring in the acquired form.
Therefore, a detailed analysis of prostate cancer epidemiology and evaluation of risk factors can help to understand the connection between genetic mutations and the role of the environment in triggering these mutations and/or favoring tumor progression.
Increased understanding of the etiology and causative risk factors of prostate cancer will provide ways to identify at-risk males and support the development of effective screening and prevention methods.
Epidemiology
Based on GLOBOCAN 2018 estimates, we have evaluated worldwide prostate cancer incidence and mortality rates, as well as analyzed incidence and mortality, temporal trends and survival rates.
Incidence
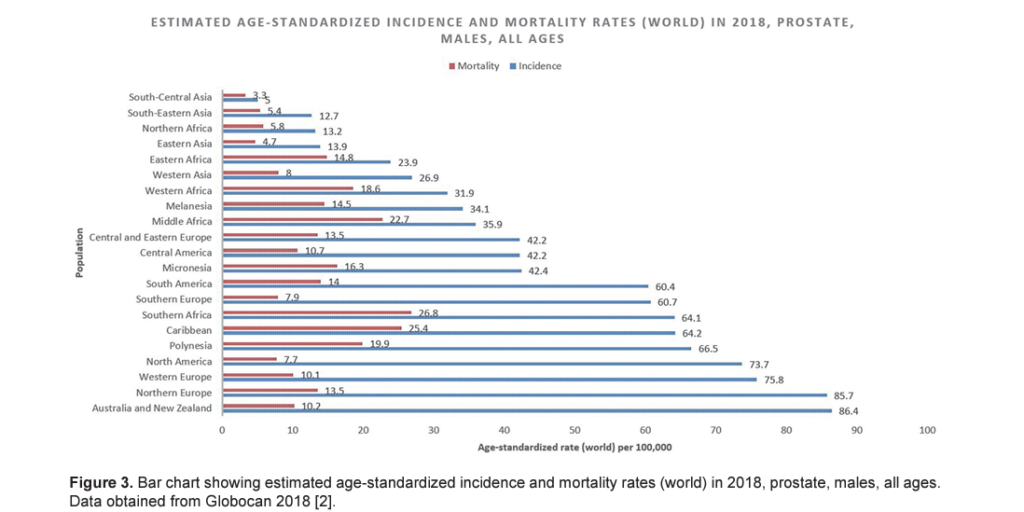
The incidence rate of prostate cancer varies across the regions and populations ( Fig. 1 ) [ 2]. In 2018, 1,276,106 new cases of prostate cancer were registered worldwide, representing 7.1% of all cancers in men [ 1]. Prostate cancer incidence rates are highly variable worldwide. The age-standardized rate (ASR) was highest in Oceania (79.1 per 100,000 people) and North America (73.7), followed by Europe (62.1). Conversely, Africa and Asia have incidence rates that are lower than those from developed countries (26.6 and 11.5, respectively) [ 2]. Differences in incidence rates were 190-fold between the populations at the highest rate (France, Guadeloupe, 189.1), and the populations with the lowest rate (Bhutan, 1.0).
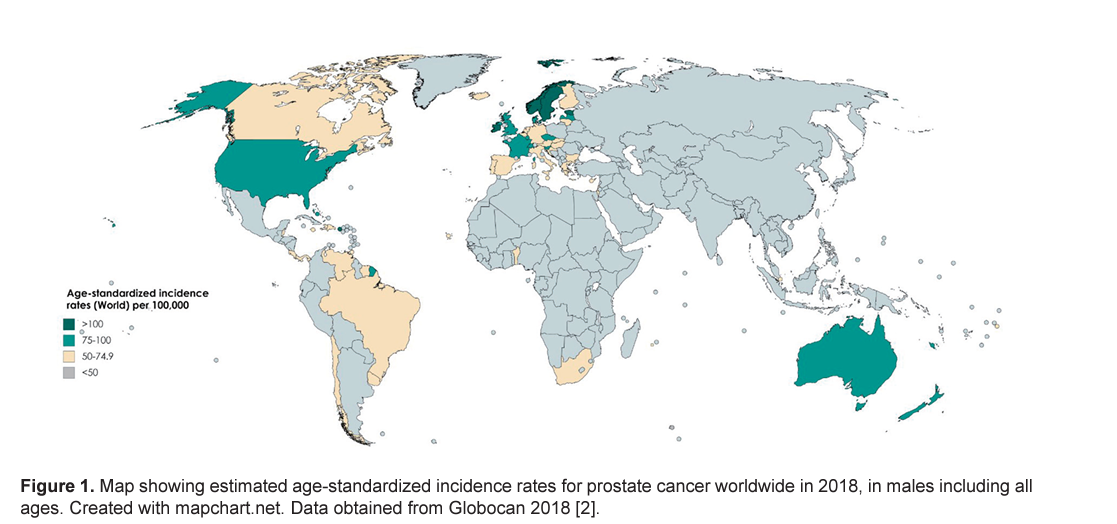
Research has shown that African-American men have the highest incidence of prostate cancer worldwide and more likely to develop disease earlier in life when compared to other racial and ethnic groups [ 17]. This is reflected in data not only for African-American men, but also for Caribbeans, and Black men in Europe, suggesting that they possess a common genetic background more prone to the development of the cancer. Of note, Chu et al [ 18] reported that incidence rates of prostate cancer were as much as 40 times higher among African-American men than those in Africa. These differences suggest that environmental factors also play an important role in the etiology of the prostate cancer and variations in incidence may be due to underdiagnosis, differences in the screening methods and disparities in healthcare access.
Mortality
International mortality rates for prostate cancer vary considerably worldwide ( Fig. 2 ) [ 2]. In 2018, the highest mortality rates were recorded in Central America (10.7 per 100,000 people), followed by Australia and New Zealand (10.2) and Western Europe (10.1) [ 2]. The lowest rate was reported in the countries of Asia (South-Central, 3.3; Eastern, 4.7 and South-Eastern, 5.4) and Northern Africa (5.8) ( Fig. 3 ) [ 2]. One-third of the deaths for prostate cancer occurred in Asia (33.0%, 118,427 of deaths), followed by Europe (29.9%, 107,315 of deaths). The mortality rate of prostate cancer rises with age, and almost 55% of all deaths occur after 65 years of age [ 2].
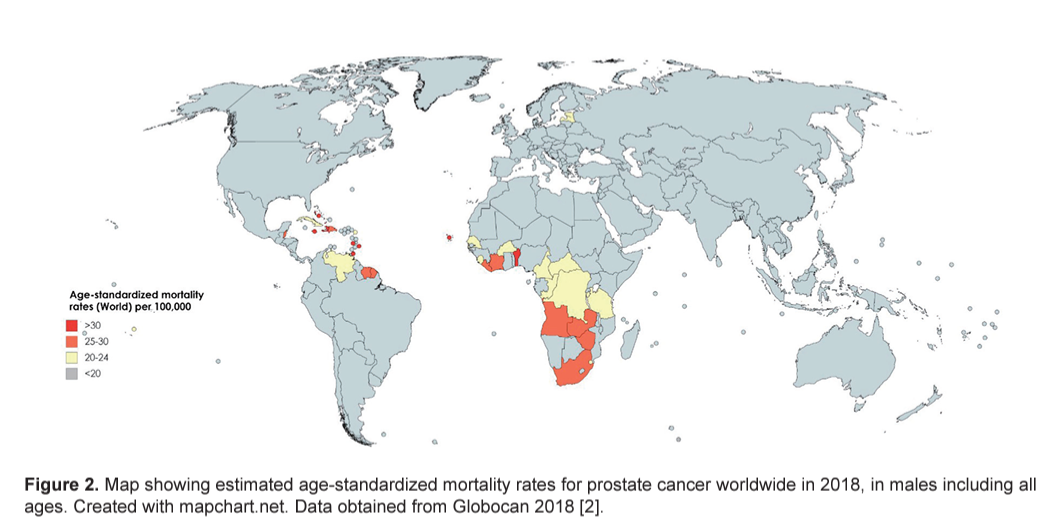
US Preventive Task Force (USPSTF, 2018) has reported that there is a potential benefit of decreasing deaths from prostate cancer in men aged 55–69 years with PSA screening [ 19]. However, for men above 70 years of age for all races, the data are less convincing [ 20].
African-American men have the highest prostate cancer incidence and mortality rates. This suggests not only that African-American men may possess some specific genes that are more susceptible to mutations in prostate cancer, but mainly that these mutations are associated with a more aggressive type of cancer. However, a study conducted by Oliver in 2007 [ 21] reported that African-American men were less likely to identify early symptoms of prostate cancer correctly than Caucasian men.
Trends
Temporal trends of prostate cancer incidence and mortality varied significantly internationally during the past years, and they seem tightly correlated to the adoption of PSA testing for early detection of the disease especially in Western countries [ 22].
Interestingly, a trend towards an increase of prostate cancer incidence worldwide with 1,017,712 new cases (+79.7% overall change) up to 2040 is estimated ( Table 1 ) [ 4]. The highest incidence of prostate cancer will be registered in Africa (+120.6%), followed by Latin America and the Caribbean (+101.1%) and Asia (100.9%). On the contrary, the lowest incidence will be registered in Europe (+30.1%). This increase in the incidence rates appears to be related to an increased life expectancy. Increasing incidence rate trends in developing countries is likely due to improved access to medical care as well as increased documentation and reporting of cases. Finally, the fact that incidence rates are increasing in those regions where PSA testing is not routinely used suggests that this phenomenon reflects westernization of the lifestyle including obesity, physical inactivity and dietary factors [ 25].
Table 1
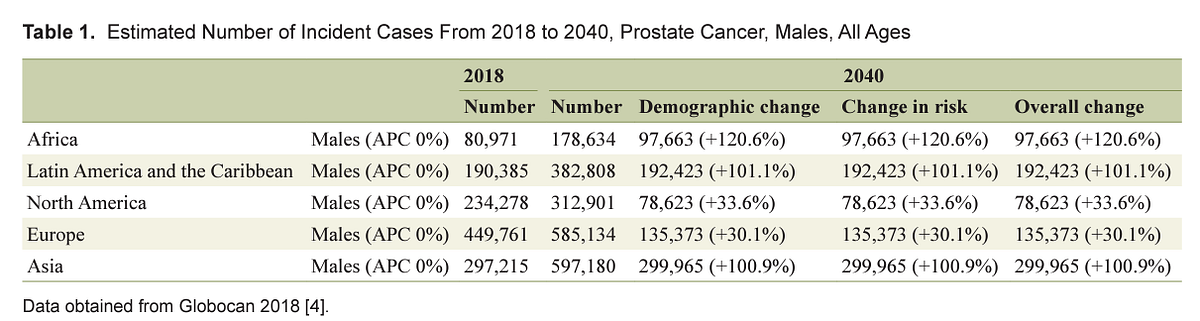
Number Number Demographic change Change in risk Overall change
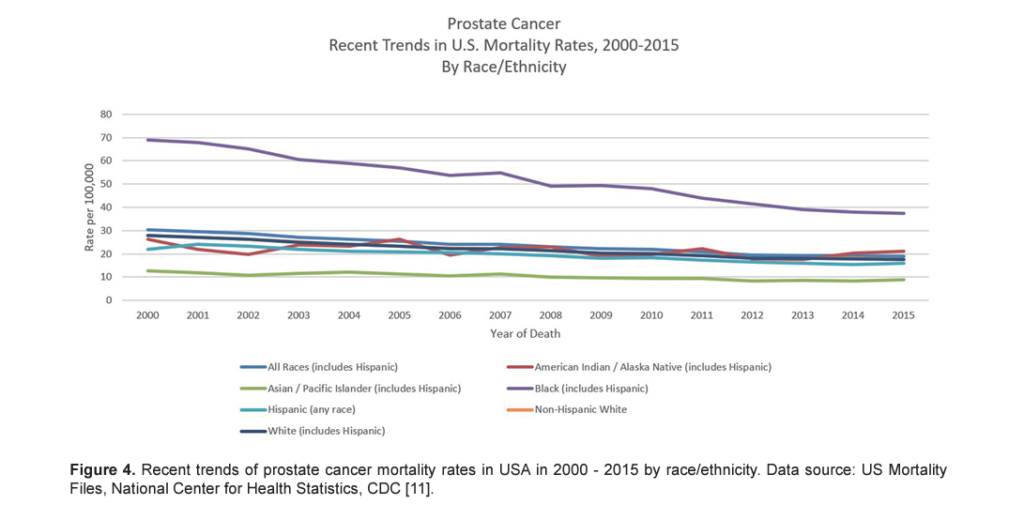
Prostate cancer mortality rates in most western countries including North America as well as in Western and North Europe have been steadily declining [ 22, 25]. Although the reasons are not clear, it may reflect both early detection and improved treatment [ 26– 28]. However, in the USA, a recent randomized controlled trial failed to demonstrate benefits of PSA testing in decreasing prostate cancer deaths, although another research study done in Europe showed benefits of PSA testing [ 29, 30]. When ethnicity-specific trends were analyzed, it was observed that the decline in mortality in African-American men was greater than that in White men between 2001 and 2015 ( Fig. 4 ) [ 11, 20]. Negoita et al documented that improved and newer modalities of detection and treatments and improved treatment of resistant and metastatic prostate cancer may justify this trend [ 20].
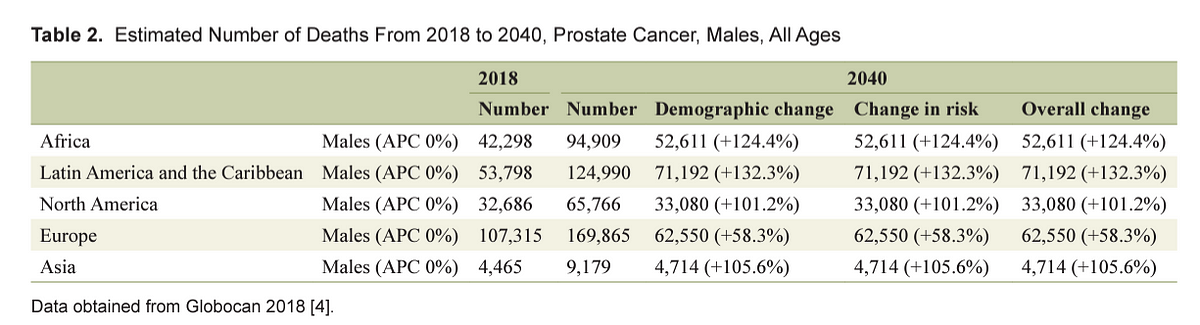
From 2018 to 2040, it is estimated that mortality will double with 379,005 deaths worldwide [ 4]. The highest mortality rate is estimated to be in Africa (+124.4%), followed by Asia (116.7%), while the lowest incidence will be registered in Europe (+58.3%) ( Table 2 ) [ 4]. The above finding is not surprising due to the limited resources for screening and detection of prostate cancer which increases the odds of it being detected during the late stages. Furthermore, considering that medical care and assistance is not widely accessible in developing countries, this may provide a possible explanation for the high mortality despite the lower incidence.
Table 2
Number Number Demographic change Change in risk Overall change
Survival
Despite in the last decades, science has made so much progress to unveil molecular mechanisms and risk factors involved in the prostate cancer, it still is the second leading cause of cancer mortality among males in the USA [ 32]. Finally, the general idea for all types of cancers is that the earlier they are caught, the earlier they can be successfully treated remaining the patients disease-free. However, because the majority of prostate cancer have a slow and often indolent course (defined as “low-risk” tumor), men can avoid immediate treatment (and prospective side effects) while safely undergoing active surveillance or watchful waiting.
Etiology and Risk Factors
The etiology of prostate cancer is the subject of numerous studies and remains largely unknown compared to other common cancers. The well-established prostate cancer risk factors are advanced age, ethnicity, genetic factors and family history [ 33– 35]. Other factors positively associated with prostate cancer include diet (increased consumption of saturated animal fat and red meat, lower intake of fruits, vegetables, vitamins, and coffee), obesity and physical inactivity, inflammation, hyperglycemia, infections, and environmental exposure to chemicals or ionizing radiation [ 34, 36– 40].
Age
Prostate cancer is the most commonly diagnosed malignancy among elderly males [ 1]. Indeed, an increasing number of senior men are diagnosed with prostate cancer due to increasing life expectancy and the increased use of PSA screening. It was observed that the risk increases especially after 50 years of age in White men who have no family history of the prostate cancer, and after 40 years of age in Black men or men with a familial history of prostate cancer [ 14].
Scardino reported that almost 30% of men over 50 years of age, who died for causes other than prostate cancer, were found with histological evidence of prostate cancer at the moment of autopsy [ 41]. Indeed, due to its indolent course, elderly men who have concurrent severe co-morbid disease during their lifetimes are more likely to die from other related health conditions or other diseases rather than from prostate cancer.
Ethnicity
Prevalence of prostate cancer highly varies among different racial groups. In the USA, the lowest incidence is observed in American Indian/Alaska (46.9) Native and Asian/Pacific Islander (52.4), followed by White (93.9). The highest incidence rate is seen in African-American men (157.6) [ 11].
Several studies proposed that genetic predisposition might play a role. African-American men have the more common chromosome 8q24 variants, which have been shown to be associated with increased prostate cancer risk [ 46– 49]. Some research studies have also demonstrated that African Americans have a high rate of variations in genes that suppress tumors such as EphB2 [ 50] or that regulate cell apoptosis such as BCL2 [ 51]. Furthermore, African-American men display a more aggressive form of the disease, which has also been associated with genetic and biologic differences, although lack of adequate screening and delayed presentation was not excluded too [ 42].
Family history and genetic factors
Gene linkage studies reveal major susceptibility loci for prostate carcinoma on genes in seven different loci. Chromosome 1q24–25 that is referred as HPC1 gene encodes the enzyme ribonuclease L (RNASEL) [ 56], which is involved in the innate immune defense mechanisms and the interferon (IFN)-mediated signaling [ 57]. It plays an important role in reducing antiviral activity and the regulation of apoptotic cell death [ 58]. Of note, analysis of human prostate cancer samples from patients with RNASEL mutations showed the presence of retrovirus unveiling the importance of antiviral defenses to prostate cancer development [ 59]. Moreover, detection of retroviral infections in some cases of prostate cancer also showed the potential connection of chronic retroviral infection and consequent tissue inflammation with cancer initiation [ 60, 61]. Another HPC gene ( HPC2/ELAC2) was identified on chromosome 17p11 and encodes a protein with poorly understood function [ 62], ELAC2, which is involved in prostate cancer development by binding SMAD2 that up-regulate proliferation through activation of TGF-beta signaling pathway [ 63]. The third identified HPC gene is macrophage scavenger receptor 1 ( MSR1), which resides on chromosome 8p22 [ 64]. However, considering the low penetrance of this allele, several studies failed to confirm its association with HPC [ 65, 66]. Additionally, a subset of HPC was found to occur in men with BRCA1 and 2 mutations that showed a clinically aggressive form of prostate cancer [ 67]. Moreover, BRCA2 mutations were correlated with a higher incidence of prostate cancer, and PALB2, BRCA2-interacting protein, was involved in familial prostate cancer [ 68].
Diet
Dietary factors may play an essential role in the development of prostate cancer as evidenced by several studies on immigrants moving from developing countries (low-risk areas) to industrialized countries (higher risk), that showed how the change to a “westernized” lifestyle induced a shift towards an increased prostate cancer incidence.
For example, Chu et al [ 18] reported that when compared to those in Africa, the incidence rate of prostate cancer among African Americans was as high as 40 times, while Hsing et al in 2000 [ 72] showed that compared to men living in China, the prostate cancer incidence was 16-fold higher for Chinese men living in the USA, suggesting that environmental factors play an important role.
There are multiple evidences that certain foods are associated at higher risk, while others are even protective.
Saturated animal fat
Multiple ecological studies have shown a positive correlation between prostate mortality and per capita intake of meat, fat and dairy products [ 73, 74]. A recent case-control study in patients less than or equal to 60 years found that high intake of total fat was associated with a statistically significant increase in prostate cancer risk [ 75].
There are several biological mechanisms that are thought to be involved between saturated animal fat intake and prostate cancer risk: 1) promoting prostate carcinogenesis via androgen; 2) increasing levels of reactive oxygen species (ROS) and increasing leukotrienes and prostaglandins levels from lipid metabolism; and 3) increasing basal metabolism, insulin growth factor and tumor proliferation.
High-calorie intake of saturated animal fat has shown to increase the growth of prostate cancer cells by increasing the circulating levels of androgens [ 76, 77]. Furthermore, randomized cross-over studies involving low-fat and high-fat diets showed that the level of androgen is lower post-prandial as well as in vegetarians [ 78]. Finally, several studies reported that alteration of lipid levels undergoing to a low-fat diet reduces testosterone levels [ 79– 81].
Excessive fat increases oxidative stress and ROS levels that attack the cells causing peroxidation and eventually DNA damage. A role for lipid metabolism and its metabolite have also been observed in mice and found that dietary fat is an important modulator of prostate cancer growth. For example, while some studies did not find any difference in terms of tumor growth and survival of mice placed on a Western diet, other studies showed a delay in cancer cell growth in mice with low-fat corn-oil diets, suggesting that the amount and type of fat are critical [ 82].
Mechanistically, corn-oil may promote cancer growth via the linoleic acid, the most abundant omega-6 fat in the oil. Arachidonic acid which is a metabolite of linoleic acid gives rise to the formation of several pro-inflammatory prostaglandins (PG), including PGE2 that promotes cell proliferation, and 5-hydroxyeicosatetraenoic acid that is produced by the action of 5-lipoxygenase, which is found to be increasingly expressed in malignant prostate cancer. Hence, a decrease in omega-6 fatty acid intake can decrease cancer growth. As opposite to omega-6 fats pro-inflammatory effect, omega-3 fats are found beneficial against cancer growth [ 83].
Red meat
Dietary meat intake has been associated with prostate carcinogenesis by correlating cancer incidence and mortality with per capita meat consumption [ 84]. Rohrmann et al [ 85] showed that men consuming five or more servings of processed meat per week had a higher risk of prostate cancer when compared with men who consume one or fewer servings per week. In African-American men, there was no association observed with high consumption of red meats and increased prostate risk. However, there was a 20% increased risk for non-advanced prostate cancer in those consuming red meat cooked at high temperature [ 86]. Cooking at higher temperatures (125–300 °C) causes the formation of aromatic hydrocarbons and mutagenic heterocyclic amines [ 87, 88]. Grilled or barbecued meat can result in the formation of N-nitroso compounds that can result in lipid peroxidation and DNA damage by the production of free radicals [ 89, 90].
Calcium, milk and dairy products
Dairy foods have generally been associated with an elevated prostate cancer risk [ 85, 91– 94]. Both calcium from supplements and dairy put male at high prostate cancer risk. Greater than 2,000 mg per day of calcium was associated with a greater risk of prostate cancer. The Health Professional Follow-Up Study examined the diet of 47,885 men and looked closely at participants’ consumption of animal food, protein and calcium [ 95]. After a 24-year follow-up, prostate cancer was diagnosed in 5,861 men, which was associated with high calcium intake [ 96].
Vegetables
Although conflicting results have been generated regarding dietary fat, a strong relationship was found between intake of Crucifers or Brassica vegetables (broccoli, Brussels sprouts, cauliflower, cabbage, and turnips) and reduced prostate cancer risk. Crucifers have anticancer properties mediated by phenyethyl isothiocyanate, sulforaphane, phytochemicals and indole-3-carbinol [ 97]. Some studies in the USA on a diet rich with broccoli have shown evidence for the protective effect of Brassica vegetables against prostate cancer [ 98]. However, some other studies revealed no anticancer capabilities of Brassica vegetables [ 99– 102].
Dietary soy and green tea
Prostate cancer incidence is significantly lower in Asia when compared to North America, which has prompted research interest in the potential chemo-preventive action of soy and green tea which are a part of the diet in Asia. Decreased risk of prostate and several other cancers has been seen with consumption of soy and green tea [ 103– 106]. Catechins found in green tea and isoflavones in soybeans have anticarcinogenic properties, and they inhibit different phases of carcinogenesis [ 107, 108] and metastasis [ 109– 111]. Additionally, green tea polyphenols cause a reduction of IGF-1 levels [ 112– 114].
Vitamin and mineral supplements
Vitamin D
An inverse relationship was observed between sunlight, or UVB exposure, and incidence of prostate cancer [ 126, 127], suggesting that vitamin D deficiency might increase prostate cancer risk development [ 128]. Similarly, discoveries were made by Barnett and Beer [ 129] who found that people living in “sunny” countries were at lower risk of developing secondary solid cancer after melanoma compared to people living in “less sunny” countries.
The incidence of prostate cancer in African-American men is twice that of Caucasians, suggesting that race might play a role. There might be a role for vitamin D deficiency in this as UV radiation is blocked in darkly pigmented skin due to high melanin levels and this mechanism inhibits the conversion to vitamin D3 [ 130].
Biochemical evidence supports a role for vitamin D in prostate growth [ 131, 132]. Cell proliferation and invasion can be inhibited by vitamin D and its analogs, and stimulate cellular differentiation and apoptosis in prostate cancer cells as well as in tumor progression in animal models [ 132– 134]. These findings provide a strong rationale for the use of vitamin D analogs as therapeutic agents for prostate cancer in a case that androgen deprivation therapy has failed [ 135]. Early clinical trials with 1α,25(OH) 2D 3 revealed serious side effect such as hypercalcemia and hypercalciuria associated with its systemic administration [ 136, 137]. Screening of several thousand vitamin D analogs identified a more potent and less calcemic compound compared to 1α,25(OH) 2D 3 when was tested in nude mice to inhibit human prostate cancer cells growth [ 133, 134]. Further studies are needed to assess the use of vitamin D analogs as a chemopreventive or therapeutic approach in prostate cancer.
Vitamin E
Vitamin E is a vitamin which is fat soluble. Vegetable oils, egg yolks, and nuts are the important dietary sources of vitamin E. Tocopherols present in vitamin E have both potent cellular anti-oxidant with anticancer properties [ 54, 138]. Studies investigating the relationship between vitamin E and prostate cancer risk have shown contradicting results. The ATBC trial showed that in men who smoked supplementing daily vitamin E was not able to reduce the incidence of prostate cancer [ 139]. In another large clinical trial (SELECT trial), vitamin E supplementation did not show any benefit in 31,000 men with incident prostate cancer [ 140].
Selenium
Selenium is an essential micronutrient. It is found in the plants like tubers, cereals and legumes and animal products like meat, eggs, and seafood in the form of selenomethionine and selenocysteine. It has been inversely associated with several cancers, including prostate cancer.
Several studies have shown a 50–60% risk reduction of developing prostate cancer when comparing high selenium consumption to low selenium consumption [ 141, 142]. The NPC trial showed a 50% reduction of incidence of prostate cancer among men that were taking selenium supplementation [ 143]; however, SELECT trial did not report any beneficial effect of administering selenium alone or combination of selenium with vitamin E [ 140]. The different outcomes could result from the utilization of two different forms of selenium: selenized yeast in the NPC, whereas selenomethionine in the SELECT. These forms differ significantly in their biological effects, and it was shown they have different mechanisms of action. Selenomethionine acts on prostate cancer cells and induces cell cycle arrest [ 144, 145]. It can also act by inducing apoptosis and inhibiting angiogenesis [ 145, 146]. Methylseleninic acid acts via a caspase-mediated pathway and induces apoptosis [ 147].
Interestingly, Chan et al [ 148] emphasized the role of genotype with respect to the effectiveness of selenium intervention. This study revealed that high selenium might be protective against an aggressive form of prostate carcinoma in men with the AA genotype of superoxide dismutase (SOD)-2 and increase the chances of having a worse tumor in men with a V allele. These data unveil the potential risks and benefits associated with selenium intervention in prostate cancer and may, in part, explain the conflicting results from other studies.
Folate and vitamin B12
Low folate and vitamin B12 can lead to altered methylation and lead to cancer development as these essential vitamins participate in DNA methylation, synthesis and repair [ 149]. In vitro [ 150], in vivo studies [ 151] and genetic studies [ 152, 153] on prostate cancer showed the role of folate in the development of an aggressive form of prostate cancer. Furthermore, elevated serum concentration of folate was associated with an increased proliferation of prostate cancer cells in some prostate samples collected from patients who underwent radical prostatectomy [ 154].
However, a recent meta-analysis reported that higher concentrations of vitamin B 12 and folate have a modest 12% increased risk of prostate cancer [ 155]. In people who have prostate cancer, available data do not show an effect of consumption of folate on disease progression [ 156] or survival [ 157]. In conclusion, the association of folate and vitamin B 12 with prostate cancer is unclear and requires further investigation.
Alcohol consumption
The relationship between alcohol use and several types of human cancers, including prostate cancer, has been since long observed [ 158]. Heavy alcohol abuse (> 15 g ethanol/day, or more than three drinks per day between wine, liquors or beer) may be a possible risk factor for prostate cancer and other cancers [ 159]. However, several cohort studies have suggested a weak correlation between alcohol intake and prostate cancer mortality [ 160– 163], while others did not find any relation with increased risk [ 164]. As opposite, Dennis et al reported a significant relationship between higher alcohol intake and prostate cancer risk with a relative risk (RR) ranging from 1.05 to 1.21 for one or four alcoholic drinks per day, respectively [ 165, 166].
Coffee
Coffee consumption has been inversely associated with increased prostate cancer risk.
Observational studies and some animal studies have revealed an association between long-term coffee drinking and improved glucose metabolism as well as insulin secretion [ 167].
Consistently, a reduced risk of type 2 diabetes was observed in those patients who reported higher consumption of coffee.
Obesity, insulin and physical activity
The possible explanation is that most of the time obese men present with alteration of circulating levels of metabolic and sex steroid hormones, which are known to be involved in prostate development as well as oncogenesis [ 178].
Exercise is supposedly one of the easiest modifiable risk factor to manage in a way to obtain many benefits and relatively few side effects when it comes to prostate cancer prevention.
Indeed, Keogh and McLeod found that veterans who exercised had a significantly lower risk of prostate cancer [ 184]. Prostate cancer patients who are committed to exercise display lower PSA levels and delay in initiating androgen deprivation therapy (ADT) by 2 years compared with less active peers and have a lower risk of high-grade disease, other than having a greater quality of life and less fatigue [ 184].
Cigarette smoking
Active and passive exposures to cigarette smoke are considered carcinogenic for many human cancers [ 185].
Sex hormones
There is a large body of both historical and modern data supporting a role for androgens in prostate cancer pathogenesis and progression, also known as the “androgen hypothesis”. In 1941, Huggins and Hodges proposed that prostate cancer growth was driven by androgens, after observing the benefits of castration in prostate cancer patients [ 192]. Several in vitro data obtained with well-differentiated prostate cancer cell lines showed that they respond to androgen stimulation and undergo apoptosis upon androgen withdrawal [ 193, 194]. Likewise, in vivo studies showed that androgens promote tumorigenesis and xenograft growth in animal models, and tumor regression is seen upon androgen deprivation [ 195, 196]. Clinically, ADT remains a mainstay in prostate cancer treatment, especially in advanced disease [ 197]. Even though preclinical studies supported a role for androgens in prostate cancer pathogenesis, clinical data are still controversial [ 198].
Several evidences that the androgen pathway is one of the most important signaling mechanisms involved in prostate cancer come from gene linkage analysis, which reveals a significant association between prostate cancer risk and single nucleotide polymorphisms (SNPs) in the genes encoding enzymes involved in the synthesis of testosterone and dihydrotestosterone (DHT): hydroxysteroid (17-beta) dehydrogenase-1, hydroxy-delta-5-steroid dehydrogenase [ 199– 202], 5α-reductase-1 [ 203] and -2 [ 204– 206], and CYP17, CYP3A4, CYP19A1 [ 207]. There is also an association between prostate cancer and the variants of androgen-responsive genes — kallikrein family, hK2 and PSA [ 208– 210], and microseminoprotein [ 211– 213], as well as genes involved in estrogen receptor signaling — estrogen receptors α [ 214] and β [ 215, 216]. Further studies are needed to understand how those gene variations influence prostate cancer incidence.
Although the positive role of androgens on prostate cell growth has been established, some studies found that in prostate cancer patients, the testosterone and DHT levels were low, suggesting that non-androgenic hormones, including estrogens, insulin and vitamin D may be involved in the prostate carcinogenesis. Several studies have demonstrated that estrogen, including the natural hormone E2, induces multiple forms of genetic lesions such as chromosomal alterations, DNA damage, gene mutations, and microsatellite instability, strongly indicating that estrogen may serve as a carcinogen in the development of prostate cancer [ 217, 218].
Insulin and insulin-like growth factor
Conversely, several other studies reported a protective effect of hyperglycemia or type II diabetes against high-grade or more advanced prostate cancer [ 224– 226]. For several decades, glucose has been documented as an important source of energy for rapid tumor cell proliferation [ 227]. Evidence from clinical and genetic studies has also linked the hyperglycemic environment to carcinogenic processes such as apoptosis, oxidative stress, DNA damage and chronic inflammation, which may drive the aggressiveness and progression of cancer [ 228– 231]. Serum glucose is directly controlled by insulin, thus higher glucose level induces insulin secretion from pancreatic β cells. Lehrer et al showed that patients with high-risk prostate cancer had higher insulin levels [ 171]. In addition, diet-induced hyperinsulinemia was associated with increased tumor growth in a xenograft model [ 232]. Finally, the high level of circulating insulin decreases the production of insulin-like growth factor (IGF-1)-binding proteins, increases the level of IGF-1 and increases the production of advanced glycation end products, which promote carcinogenesis [ 233].
Chronic inflammation and prostatitis
Patients with elevated PSA often present with intraprostatic inflammation detected with biopsies [ 245]. Recently, an inflammatory effector, pentraxin 3, has been identified as a biomarker for predicting tumor progression due to prostatic inflammation in prostate cancer patients [ 246].
Chronic inflammation causes proliferative inflammatory atrophy (PIA) [ 174], which may develop prostatic intraepithelial neoplasia (PIN) a well-known precursor of prostate cancer [ 247]. Consistently with the tight connection between chronic inflammation and high-grade prostate cancer [ 243], several SNPs in genes involved in inflammation, such as cyclooxygenase (COX-2) [ 248, 249], interleukin-1 (IL-1) [ 250], IL-6 [ 251, 252], IL-8 [ 250] and IL-10 [ 250, 253], tumor necrosis factor-α (TNF-α) [ 254] and Toll-like receptor-4 (TLR4) [ 253, 255– 257] were associated to prostate cancer risk.
Prostatitis is the inflammation of the prostate gland that is hard to diagnose because it is often asymptomatic [ 258]. Notably, men with symptoms of prostatitis are more likely to be diagnosed with prostate cancer as a result of the increased prevalence of biopsy [ 259].
Development of prostatitis is induced by one or a combination of factors including infections, chemical and physical trauma, and diet. Chemical irritation because of urine reflux, or abnormal flow of urine from the bladder back through the ureters, may cause chronic inflammation in the prostate [ 263]. Non-sexually transmitted pathogens such as E. coli and Propionibacterium acnes can cause acute and chronic prostatitis [ 264, 265]. Also, many sexually transmitted organisms, including Neisseria gonorrhea [ 266] and Chlamydia trachomatis [ 267], can induce chronic infection and inflammation, that potentially increase the risk of developing prostate cancer [ 268].
It appears clear that inflammation is the ubiquitous factor associated with increased risk of prostate cancer, independently of its source (either pathogens or environmental factors).
Finally, the several signaling pathways involved in the inflammatory process that modifies prostate microenvironment and the complexity of biological events linking inflammation to prostate cancer progression and metastatic spread provide a broad range of promising targets for pharmaceutical treatments.
Sexually transmitted disease (STD)
Several epidemiologic studies evidenced that factors related to sexual behavior and STDs may be associated with prostate cancer [ 269].
A large population-based case-control study among African-American and white men, revealed an elevated risk of prostate cancer among men with a history of gonorrhea or syphilis [ 268]. HPV, which occurs in human prostate cancer and benign prostatic tissue [ 276], has been shown to transform human prostate cells in vitro. Furthermore, seropositivity for HPV-18 and HPV-16 has been associated with subsequent prostate cancer in a Finnish cohort study [ 277, 278], but a small case-control study of HPV-16 and HPV-11 [ 279], and a large population-based case-control study [ 268] showed little evidence of risk. Finally, a recently published meta-analysis showed a weak association between HPV-16 and prostate cancer and no association for HPV-18 [ 280].
To our knowledge, only three studies have been published so far investigating the association between Trichomonas vaginalis infection, a common cause of vaginitis in women, and prostate cancer risk. Trichomonas vaginalis can also infect men, where it may cause asymptomatic urethritis and prostatitis. In particular, its frequent asymptomatic presentation may make it possible to persist untreated and ascend to the prostate, where it can establish foci of chronic inflammation that may eventually lead to prostate cancer [ 275]. Mechanistically, Trichomonas vaginalis infection causes adherence of the protozoan to epithelial cells by decreasing the expression of anti-apoptotic genes; it also alters the production of IL-6 and monocyte chemotaxis proteins. Whereas an association between Trichomonas vaginalis serostatus and increased risk of prostate cancer was found, the same connection between seropositivity for this pathogen and progression to death from prostate cancer was not demonstrated [ 281].
Medications
Despite the knowledge gained over the years about the etiopathogenesis of prostate cancer and the known high risk for men to be diagnosed with the disease during their lifetime, effective chemo-preventive agent that can safely be administered to impact the lives of men is still missing positively. The role of testosterone and pro-inflammatory pathways in the pathogenesis of prostate cancer provided some clues about the possibility to use inhibitors of testosterone endogenous production and inflammation.
5-α reductase (5-AR) inhibitors
By far the most promising and well-studied chemo-preventive agents are finasteride and dutasteride, which are inhibitors of 5-AR enzyme that converts testosterone into dihydrotestosterone, the most prevalent and potent androgen in prostate tissue, which is responsible for embryologic development [ 282] and growth of the prostate as well as promotion of prostate cancer [ 283].
Finasteride and dutasteride are effectively used for the treatment of benign prostatic hyperplasia [ 284– 286] and were studied in clinical trials as potential chemopreventive agents.
Finasteride was studied in the prostate cancer prevention trial (PCPT) and its use was associated with a 25% reduction in prostate cancer incidence after 7 years [ 287]. Dutasteride was studied in the REduction by DUtasteride of Prostate Cancer Events (REDUCE) trial, and the results showed that men treated with dutasteride had a 23% reduction in prostate cancer incidence after 4 years [ 288]. However, the results of these two trials were largely criticized for many aspects, including results from biopsies performed towards the end of the study as opposed to biopsies that are done when patients have elevated PSA or DRE abnormalities, the capability to prevent only low-grade cancers that will unlikely lead to death. Most importantly, Food and Drug Administration (FDA) Oncology Drugs Advisory Committee (ODAC) re-analyzed the data from PCPT and REDUCE trials and confirmed that the results do not strongly support any preventive effect on high-grade prostate cancer and therefore their use in therapy as chemo-preventive agents is not recognized yet.
The Reduction by Dutasteride of Clinical Progression Events in Expectant Management of Prostate Cancer (REDEEM) study reported that dutasteride may provide a useful adjunct to “active surveillance” for management of prostate cancer, because it delayed the time to prostate cancer progression, increased the percent of men with no detectable tumor and improved cancer-related anxiety [ 289].
However, whether the effectiveness of 5-AR inhibitor therapy is influenced by certain patients’ features, like specific clinical conditions or genetic variations, was not evaluated yet. Therefore, identification of these subgroup of patients who may then undergo clinical trials with 5-AR inhibitors would address this intriguing question.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin (ASA)
There is rapidly growing evidence for the impact of NSAIDs on cancer [ 290]. At the cellular level, NSAIDs target cyclooxygenase (COX), particularly, the isoform 2 (COX-2) is expressed in inflammatory cells of the prostate and in PIA, a precursor of prostate cancer [ 174, 243]. A recent meta-analysis that included 20 observation studies with a total of 25,768 individuals evaluated the efficacy of NSAIDs in reducing prostate cancer risk [ 291]. The clinical data indicated that there was a statistically significant protective effect as revealed by the risk reduction at 5% for ASA and 8% for other NSAIDs. In addition, another study revealed that they were only effective for patients > 60 years of age [ 292].
Several experimental studies have documented that COX-2 overexpression in prostate cancer can be effectively targeted by COX-2 selective inhibitors such as celecoxib [ 291].
Several epidemiological and experimental evidence has demonstrated an inverse relationship between ASA use and prostate cancer, particularly after 5 or more years of use in men with metastatic disease [ 293]. A population-based case-control study of 1,900 men reported that the RR of prostate cancer was significantly reduced of 18%, 21% and 24% in men who reported the use of ASA, or currently used ASA, or are long-term users of ASA, respectively [ 294]. Surprisingly, low dose of ASA was associated with the highest risk [ 294]. Finally, a multicenter study of over 90,000 documented a direct protective effect in patients ingesting six ASAs daily [ 295]. Altogether, the results of these studies have shown that the use of NSAID and ASA have a beneficial effect on the prevention of prostate cancer.
Statin
Statin medications are inhibitors of the synthesis of lipids, particularly cholesterol and recently they showed to reduce PSA levels [ 296, 297], and the risk of advanced or aggressive prostate cancer [ 298]. They are also associated with improved outcomes after radiation therapy [ 299] and radical prostatectomy [ 300], although data for the latter are conflicting [ 301].
The use of statins as a preventive agent may offer the advantage to reduce cholesterol levels and the risk of cardiac disease other than being safe. Statins effect as a secondary preventive agent was recently tested in two studies. One trial enrolled patients and randomly assigned them to simvastatin or placebo treatment before radical prostatectomy and examined changes in benign and malignant tissue in the prostate specimen [ 302]. From this study, no significative beneficial action of simvastatin was observed and its use was associated to serious adverse events (+55% vs. 18.75% of placebo group). The second trial is a phase II study evaluating the effect of atorvastatin and celecoxib on the levels of prostate cancer biomarkers, including PSA, in those patients with rising PSA levels after definitive local therapy [ 303].
In conclusion, more clinical evidence is called to prove the effective advantage of using statins for prostate cancer prevention.
Environmental carcinogens
The slow process of prostate carcinogenesis is also influenced by exposure to certain environmental factors that increase the risk of developing cancer. These include insecticides, herbicides and other organic compounds.
Agent orange (AO)
Herbicides are active chemical compounds that are used to fight plant pests. Agent orange (AO) is a mixture of two herbicides that were used as a defoliant between 1962 and 1971 in the Vietnam War and was contaminated with the toxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a putative carcinogen. Dioxins remain an area of important interest as these environmental toxins continue to be produced through chemical processing and municipal waste incineration. These chemicals can then enter the food chain through soil contamination [ 304].
In 1998, the National Academy of Science of the US recognized a positive association between herbicide exposure and many human cancers. In a review published in 2008, the authors found twice as many cases of prostate cancer among the exposed veterans of the Vietnam War compared with no-exposed veterans [ 305]. They presented with earlier diagnostic ages, high-grade tumor, independently of other modifiable risk factors. It suggests that AO is the most predictive factor not only of developing prostate cancer but also of a higher histological grade and a greater probability of metastatic disease at diagnosis. These data were later confirmed by the study of Ansbaugh et al that demonstrated a high correlation between AO exposure and risk of high-grade prostate cancer among Vietnam War veterans [ 304].
Chlordecone
The prostate cancer risk associated with chlordecone exposure was higher in those men with a family history of prostate cancer, and similar findings were reported for pesticide exposure in the Agricultural Health Study [ 309– 312]. There are two possible explanation: 1) Study subjects and their first-degree relatives may have similar patterns of exposure, which consequently lead to a statistical interaction between chlordecone exposure and family history of prostate cancer; 2) Genetic variations, such as inheritance of polymorphism in a metabolic enzyme that alters the balance between chlordecone bioactivation and detoxification in the body. In line with the latter hypothesis, previous studies have shown differences in the inter-individual liver activity of chlordecone reductase between White and Japanese men [ 313, 314].
Bisphenol A (BPA)
Another harmful compound associated with a high risk of prostate cancer is BPA.
The first evidence on the direct link between BPA exposure and human prostate cancer was reported in 2014 by two US research teams based on in vitro and in vivo experimental studies [ 320, 321]. Abnormalities of centrosome (a hallmark of malignant transformation) induced by a low level of BPA and its analogs were underlined as the potential mechanism in promoting the formation of prostate cancer [ 320, 322]. Tse et al published epidemiological evidence that cumulative exposure to BPA was associated with an excess risk of prostate cancer in the Chinese population [ 323]. The authors found a positive association of prostate cancer with intake of deep-fried food and pickled vegetable that were independent of other risk factors [ 324– 326]. Deep fried foods (e.g. meats and potato) at high-heat cooking process generate a high amount of heterocyclic amines and other mutagens and carcinogens (e.g. acrylamide and polycyclic aromatic hydrocarbons) that may be carcinogenic to prostate cells [ 325].
Vasectomy
Vasectomy is the most frequent male contraception in the USA, with approximately 500,000 procedures performed annually. Its association with prostate cancer risk was explored in case-control and cohort studies with conflicting results. Some authors found an increased risk of up to 70% with a vasectomy, while others found a lesser risk [ 189, 327, 328]. However, studies showing small RRs are not convincing as they have some potential bias and methodological shortcoming [ 329]. On the other hand, in a very well-designed study in New Zealand, the country with the highest global prevalence of vasectomy, the authors found no relationship with these subgroups [ 327].
Biological studies that show underlying mechanism/s that might explain an association between vasectomy and prostate cancer are still lacking.
Ejaculatory frequency
Over the years, there has been growing evidence of a link between ejaculation and lower chances of prostate cancer.
In 2004, the Health Professionals Follow-Up Study (HPFS) cohort reported a significant positive relation between monthly ejaculation frequency and prostate cancer risk based on 8 years of follow-up [ 330].
A major study of 2016 that involved almost 32,000 men revealed that men who ejaculated ≥ 21 times per month (EPM) had about a 20% lower chance of low-grade prostate cancer, compared with those who had ≤ 4–7 EPM based on 18 years follow-up [ 331]. A year later, a case-control study sampling a smaller group of men (2,141) from age 20 to 50 found only weak evidence of an inverse association between ejaculatory frequency in the fourth decade of life and advanced prostate cancer, which was not significantly modified by a number of new sexual partners [ 332]. In the same study, no relationship was found for ejaculatory frequency in the third and fifth decades of life.
Diagnostic radiologic procedure and ultraviolet light exposure
The radiation generated from X-ray, CT and nuclear imaging is ionizing radiation that penetrates the tissue to reveal the body’s internal organs. However, ionizing radiation can damage DNA, and although cells repair most of the damage, sometimes small area may remain altered (“misrepair” area) consequently leading to DNA mutations that may contribute to cancer development years down the road. The first study investigating the connection between low-dose ionization radiation from diagnostic X-ray procedures and risk for prostate cancer reported that exposure to a hip/pelvic X-ray significantly increased prostate cancer risk independently of other known risk factors such as family history of cancer [ 338]. However, unless men were exposed to high doses of radiation during cancer treatment in youth, any increase in the risk for cancer due to medical radiation appears to be slight. Considering that the increase in high-dose imaging has occurred only since 1980 and the effects of radiation damage typically take many years to appear, this may explain the weak association between ionizing radiation and prostate cancer risk observed thus far.
Prevention
An effective prevention strategy for prostate cancer would provide many benefits to men with a substantial positive impact on public health, including the potential to reduce the high lifetime risks of prostate cancer development, the morbidities associated with cancer treatment, especially in those newly diagnosed patients with biological indolent prostate cancer that still undergo curative-intent therapy rather than active surveillance and finally the inability to eradicate life-threatening metastatic prostate cancer.
Epidemiological data indicate a dominant role for lifestyle factors in prostate cancer development. Considering that prostatic carcinogenesis takes many decades, lifestyle modification may represent a feasible and cost-effective approach to retard prostate cancer development.
Although the data about the role of specific lifestyle factors fostering prostate cancer development have often been conflicting, most of the studies clearly evidence a diet rich in fruits, vegetables and anti-oxidant micronutrients, and poor in saturated fats and “well-done” red meats, may significantly reduce risks of prostate cancer development, as well as the risk of diseases typical of the industrialized world.
The better understanding of prostate cancer etiology represents the key to open to new opportunities for prostate cancer prevention. Several nutrients and pharmaceutical agents have been studied as potential chemoprevention candidates. Vitamin E and selenium showed promise [ 138, 141]. However, these were definitively evaluated in the SELECT, and neither agent reduced prostate cancer risk [ 140]. Vitamin D analogs, NSAIDs and toremifene (a selective estrogen receptor modulator) have all been evaluated in laboratory and/or observational studies [ 134, 339, 340]. However, vitamin D has not been formally tested in primary prevention trials, while the NSAID rofecoxib, a COX-2 selective inhibitor, could not be successfully tested because the trial was closed when the drug was withdrawn from the market after an interim safety analysis indicated that the drug was associated with increased risk of cardiovascular events within the Adenomatous Polyp Prevention on Vioxx (APPROVe) trial [ 341]. Toremifene showed a modest risk reduction in a phase II trial [ 342], but no significant risk reduction in a phase III trial [ 343].
Besides identification of molecular targets, other principles of prevention include personalized risk assessment, identification of sensitive and accurate surrogate molecular markers to serve as intermediate endpoints and identification of biomarkers that predict sensitivity to preventive agents. Furthermore, it should also emphasize personalized molecularly targeted approaches for the selection and treatment of patients with prostate cancer that result in a positive outcome and effective therapy.
Future Directions
The high global incidence of prostate cancer makes a call to strengthen the current tools available to identify trends and prevention strategies to reduce the public health impact of this disease in the future.
First, prostate cancer registries play an important role in the advancement of prostate cancer research and care. Indeed, they represent an essential source to collect information about incidence and mortality, disease characteristics at presentation, trends and risk factors, quality of care, disparities in access to treatment, long-term data related to oncologic and health-related quality of life outcomes and costs associated with management of the disease. Therefore, improvements in the quality of data, collection of tissue samples and the availability of data feedback to health care providers will increase the relevance of epidemiological studies especially when it comes to the evaluation of data collected from undeveloped countries.
Chemo-preventive strategies have been explored in several preclinical and small clinical studies to mitigate the global burden of prostate cancer and the overtreatment of indolent disease that has been associated with the broad utilization of PSA testing. However, a challenge for the future will be the translation of preclinical data into clinically useful strategies, which will require very large trials with thousands of participants, like those of the SELECT studies [ 180].
Furthermore, studies that can fill the knowledge gap regarding the higher prostate cancer incidence and mortality in African-American men compared to White men are also needed. Recently, the “Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Tumor Markers, and Social Stress” (RESPOND) study which was funded by the National Institute of Health and Prostate Cancer Foundation was carried out. The primary goals of this study were to understand how social and genetic variants contribute to the development of aggressive prostate cancer, and how those factors interact with each other. Hopefully, the increased knowledge gained within this study will provide new insights to develop positive screening and chemo-preventive strategies.
Finally, classical prognostic factors such as PSA testing, Gleason score and clinical cancer staging have demonstrated not to be always sufficient to lead to a clinically relevant cancer diagnosis. Considering that various genomic aberrations contribute to the variety in prostate cancer risk and outcome as well as drug responses and progression between patients, the identification of novel genetic biomarkers is highly required. This will undoubtedly improve cancer diagnosis, subtype identification and risk stratification. Most importantly, as we are moving toward personalized medicine, oncogenetic testing and biomarker profiling will facilitate the optimal therapeutic intervention based on the alterations observed in single patients [ 344, 345]. Clinical trials have already shown high success rate for drugs that are developed using biomarkers in patients with non-small cell lung cancer, therefore it is desirable that same results may also be achieved for the treatment of prostate cancer.
Conclusion
Prostate cancer is the most common malignancy in men, ranking second after lung cancer [ 1].
The identification of biomarkers such as PSA that are positively correlated with the diagnosis of prostate cancer revolutionized the epidemiology of this disease.
Indeed, since the introduction of PSA testing and subsequent biopsies, USA registered double of prostate cancer incidence starting from the late 1980s [ 23].
A similar increase was also reported in other countries, particularly in the western type.
Unfortunately, although it turned effective in reducing prostate cancer-specific mortality, the relevant overdiagnosis and the severe side effects of treatments advised against the introduction of PSA as a screening program.
Perhaps, the most dramatic statistic when it comes to prostate cancer incidence and mortality is the way that prevalence varies among different racial groups, with the highest prevalence in African-American men [ 14].
Both biologic and socioeconomic factors may explain this discrepancy, but which genes may be involved and how they may interact with the environment is still unknown and is a subject of studies.
In 2018, a study called “Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Tumor Markers, and Social Stress” (RESPOND) was financed by the National Cancer Institute, the National Institute on Minority Health and Health Disparities and the Prostate Cancer Foundation with the purpose of addressing those questions.
In recent years, the development of novel genetic technologies allowed for the first time a comprehensive analysis of genetic and epigenetic changes in human prostate cancer.
This information, combined with targeted functional studies, helped to identify critical signaling pathways that are casually involved in prostate cancer initiation and progression.
This information will provide an opportunity for the development of novel targeted approaches for therapeutic interventions. More research to identify genes associated with an increased risk of prostate cancer is ongoing, and researchers are collecting more insights about the impact that specific genetic changes have on prostate cancer development.
Although there are no studies that can sufficiently demonstrate the direct correlation between diet and nutrition with risk or prevention of prostate cancer development, many preclinical studies that look at links between certain eating behaviors and cancer suggest there may be a connection.
Moreover, these studies allowed identifying the underlying biological mechanisms that may explain this link.
Therefore, well-designed trials that replicate preclinical findings are warranted to validate the impact of dietary agents in prostate cancer.
Finally, future chemoprevention studies should include not only early intervention but should also emphasize personalized molecularly targeted approaches for the selection and treatment of patients with prostate cancer that result in a positive outcome and effective therapy.
Acknowledgments
None.
Conflict of Interest
None of the authors have conflict of interest.
Ethics Approval
No ethics approval needed.
Funding
No funding to disclose.
Author Contributions
Conception and design: PR. Analysis and interpretation, drafting and critical revision of the article: PR. Final approval of the article: PR.
References
See the original publication
Articles from World Journal of Oncology are provided here courtesy of Elmer Press
About the author
Hospitalist, Department of Internal Medicine, SOVAH Health, Martinsville, VA 24112, USA.
Cite
Rawla P. (2019). Epidemiology of Prostate Cancer. World journal of oncology, 10(2), 63–89. https://doi.org/10.14740/wjon1191
Originally published at https://www.ncbi.nlm.nih.gov.Epidemiology of Prostate Cancer
Prostate cancer is the second most frequent cancer diagnosis made in men and the fifth leading cause of death…www.ncbi.nlm.nih.gov












