StatNews
By Edward Chen
Aug. 23, 2022
Site Editor
Joaquim Cardoso MSc.
Health Transformation . Institute (HTI)
At Home Testing Unig
August 29, 2022
As the world continues to learn how to live with Covid-19 in the long run, scientists are testing ways to quickly tell people how well-protected they are against the virus, and whether they need another booster.
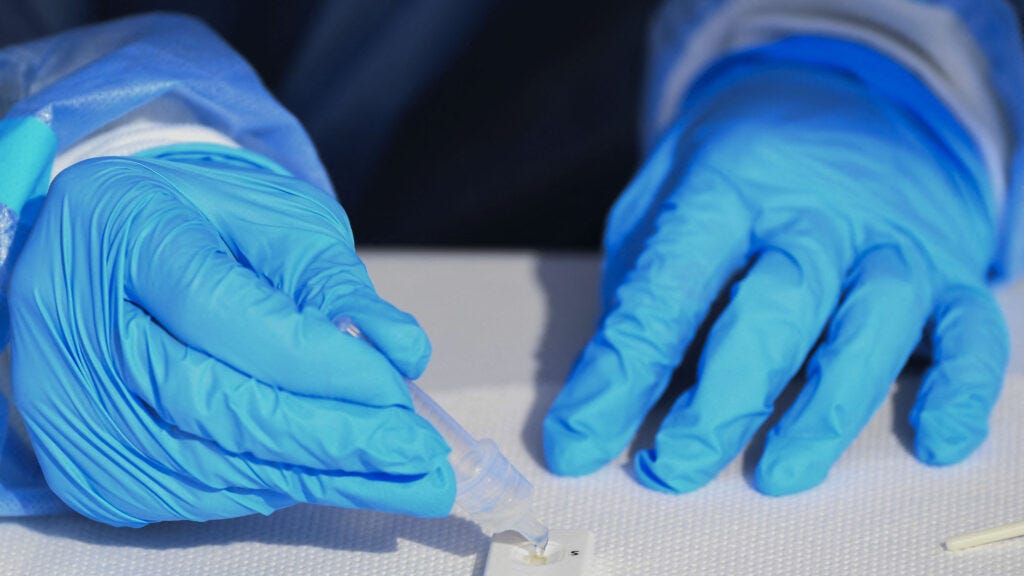
A new study, published Monday in Cell Reports Methods, presents a simple test to detect neutralizing antibodies against SARS-CoV-2, using little more than a finger prick and a testing cartridge. The approach, if it bears out in large-scale testing and receives the blessing of regulatory agencies, could one day offer a cheap, easy option to measure protection against the virus.
“People want to know, ‘Am I protected today?’
And there isn’t a tool on the market that can facilitate that answer,” said Charles Mace, a chemist at Tufts University who was not involved in the new study. “This has that potential.”
Antibodies float around in the bloodstream, waiting for a foreign invader to make it in.
When a virus does, some antibodies that are specific for parts of the virus — neutralizing antibodies — recognize and bind to it, which marks the virus for destruction and prevents it from infecting any cells. The body can naturally develop neutralizing antibodies through exposure to the virus after vaccination or an infection.
When scientists want to determine whether those neutralizing antibodies are present in a person, they take blood samples, separate out the serum, and mix it with live SARS-CoV-2 virus.
They then incubate that mixture with a human cell line and see how many of those cells die off.
Scientists can also use other viruses that have been engineered to make the spike protein and a fluorescent molecule. But both of those methods require live virus, which means training and a biosafety lab, and take about 2 days to run.
Researchers have been developing other, faster methods.
But by and large, they either require laboratory equipment or are more complicated to manufacture because they use antibodies.
The project started when Hojun Li, a hematologist and investigator at the Koch Institute, saw a bone marrow transplant patient at the Dana-Farber Cancer Institute right around the time that the pandemic hit the U.S. hard. But before the operation, they presented with symptoms for Covid-19. At the time, the only option available for doctors to rule out a Covid-19 infection was to do a blood test and examine the antibody levels. It took a week for the results to come back negative and Li to be able to proceed with the bone marrow transplant. “These are usually very urgent, urgent things to do,” Li said. “The longer you wait, the more likely whatever underlying diseases that come back cannot be cured by the bone marrow transplant.”
Li and his colleague at the time, Guinevere Connelly, started talking about how they could close that knowledge gap with Covid.
Connelly, a co-first author of the study and now a Ph.D. student at Duke University, said they quickly realized “that what would be really helpful would be a neutralizing antibody test where you could see what level of protection you may have against infection from Covid or from SARS-CoV-2.”
With collaborators, they learned new techniques to make every component of a test, from producing viral proteins at a larger-scale to cycling through a hundred different buffer formulas.
It took them a year to collect enough test samples — repeat blood draws from two people before and after vaccination, and single donations from 93 other individuals — to design and validate the test.
The result of their work is a device that looks and works like existing rapid antigen tests, which detect protein from the virus when someone is actively infected.
The new test uses a small amount of blood, obtained via a finger prick, that gets mixed with a buffer that contains the portion of the SARS-CoV-2 spike protein that binds to the ACE2 receptor on human cells.
Where a typical antigen test has two binding sites — a control line that shows the test is working properly and a test line that lights up when a person is positive for the coronavirus — the new one has three: a control line, a line turns an intense red when a person does not have neutralizing antibodies, and a line that appears when they do.
“We also like people to think about positive signals as opposed to reduction of signals because there’s just a general feeling that that’s more reliable,” said Angela Koehler, a bioengineer at MIT who worked on the new test. Indicating both the presence and absence of neutralizing antibodies, the researchers said, allows everyday consumers to snap a picture with their mobile app to know their quantitative antibody titer.
“If you have an actual number that you can measure — and neutralizing antibody concentration would be a number that you can actually measure — then you can tell what degree of protection they have and whether or not they might need a subsequent [dose] or a booster sooner rather than later,” said Li. That kind of information could be of interest to a wide range of people, but would be particularly helpful for those who have weaker immune responses, like those Li sees in the clinic. “The vast, vast majority of patients I see get bone marrow transplants,” he added. “Their immune system is just really wiped out.”

Basing booster decisions on antibody levels is not too far-fetched an idea.
In Australia, for example, antibody testing is used to determine if someone needs a 4th dose of the hepatitis B vaccine.
But Julio Delgado, the clinical pathology chief at the University of Utah School of Medicine, is more skeptical that the new test will find use among the broader population. “Even if I get vaccinated, I’m still going to get infected at some point. That happened to me,” said Delgado, who has developed laboratory-based clinical tests that measure levels of antibodies against SARS-CoV-2. “So the understanding about neutralizing antibodies has really become more of a scientific interest, not necessarily a clinical utility.”
Some experts said that any use case was overly optimistic because we don’t yet know what antibody levels translate to optimal protection.
This is complicated by the fact that different assays calculate titer levels differently. “There is not an understanding about thresholds above or below which people are going to be prone to getting infected, or prone to develop progressive disease, or prone to die,” said Delgado. “Yes, you get a number, but what does that mean?”
In part because the new test can be manufactured for less than a dollar a kit, its developers said that is exactly one of the questions their new product can answer.
“It’s really the first one that I’ve seen that can answer that question,” said Hadley Sikes, a chemical engineer at MIT who was a senior author of the research. “If there was a study design where you use this tool — you recruit a cohort, say, 5,000 or 10,000 people, you get these neutralizing antibody tests in their homes, and you follow who gets infected and doesn’t get infected — it’s an answer to a question that a lot of people, a lot of doctors, a lot of articles in the press have been raising.”
The team behind the test said they are interested in pursuing an emergency use authorization down the road and would want to work with an industry partner or foundation that has experience with regulatory applications.
But Li acknowledged that the Food and Drug Administration “has a little bit of hesitancy about anything antibody-related,” but attributed this to tests that measured all antibodies, rather than only SARS-CoV-2 neutralizing antibodies. “A lot of those tests flooded the market and a lot of them, frankly, were very, very poorly managed,” he said. Because antibody levels decline gradually, testing once a month would likely be enough. On the flip side, though, such infrequent testing needs might make it less appealing for commercial players to invest in the studies that would be needed to move the tests from a proof-of-concept to an authorized product.

A different approach on the horizon uses a costlier machine called a spectrometer to detect the Covid-fighting antibodies.
Cheng-Hao Ko, an engineer at the National Taiwan University of Science and Technology, said this would be “300 to 400 times more accurate” than the rapid test approach. Ko is collaborating with a group at Temple University to seek emergency use authorization from the FDA for that device.
That level of accuracy likely wouldn’t be necessary for the general public. “If you have a good indication of low, mid-range, and high level of antibodies, that’s probably good enough for the purpose,” said Jean-François Masson, a biochemist at the Université de Montréal in Canada.
Adolfo Garcia-Sastre, a virologist at the Icahn School of Medicine at Mount Sinai, said there’s also a potential to use this type of test as a tool to understand immunity at the population level and help guide public health decisions, for example by routinely testing samples from blood donors. “Surveillance is going to become more important, I hope, in the next year,” he said, “because it gives you an idea of what is the vulnerability.”
Originally published at https://www.statnews.com on August 23, 2022.