the health strategist
knowledge platform
Joaquim Cardoso MSc.
Chief Research Officer (CSO), Chief Editor
Chief Strategy Officer (CSO) and Senior Advisor
August 7, 2023
What is the message?
Researchers at Stanford devised a strange new molecule that could lead to drugs that arm genes and make cancers work against themselves.
Key takeaways:
- In a groundbreaking advancement in cancer research, Dr. Gerald Crabtree, a developmental biologist at Stanford University and founder of Shenandoah Therapeutics, has achieved a significant breakthrough. Collaborating with Nathanael S. Gray, a professor of chemical and systems biology at Stanford, they have developed a novel approach that holds the potential to target and destroy cancer cells by manipulating the very molecules responsible for their growth.
- The team’s findings, detailed in a paper published in the journal Nature, have generated excitement in the scientific community and could potentially serve as a foundation for future cancer drug development. While still in the early stages of research, this approach represents a promising avenue for advancing cancer therapies.
- The central idea revolves around targeting molecules within cancer cells that fuel their aggressive growth. Dr. Crabtree’s inspiration for this breakthrough came during a walk in the redwoods, where he envisioned harnessing these molecules to induce cell self-destruction.
- The team successfully designed and constructed molecules that link the mutated protein BCL6 (which drives cancer growth) with a normal cell protein responsible for activating genes.
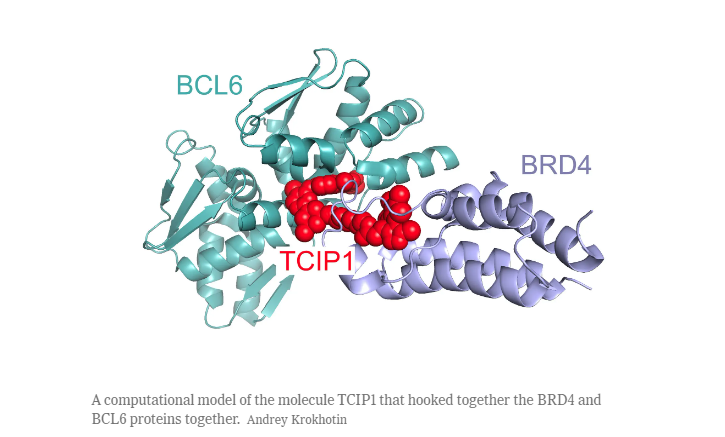
- This hybrid molecule, resembling a dumbbell, directs itself towards cell-death genes within the cell’s DNA. When it interacts with these genes, the normal protein on the hybrid molecule triggers irreversible activation of the cell-death genes, effectively causing the cancer cells to undergo programmed cell death.
- This innovative concept involves reprogramming cancer-driving proteins to instead activate cell-death pathways, essentially making the cancer cells self-destruct.
- One key advantage of this approach is its specificity. By targeting the mutated proteins that are unique to cancer cells, healthy cells are spared from damage. In laboratory experiments using cells from a blood cancer called diffuse large B-cell lymphoma, the researchers successfully activated the cell-death genes, effectively halting the growth of these cancer cells.
- The potential impact of this discovery is significant. While acknowledging that there is still a long way to go before developing a viable treatment, experts have praised the concept’s potential to revolutionize cancer therapy.
- This approach could potentially be applicable to roughly half of all cancers, which involve mutations that drive abnormal protein production. Furthermore, the treatment could be highly specific, minimizing harm to healthy cells.
- Dr. Crabtree’s work builds on two important discoveries in the field of cancer research: driver genes that fuel cancer growth and the existence of death pathways within cells. By linking these two aspects and manipulating the molecules responsible for each, the researchers have created a powerful tool to disrupt cancer cells’ survival mechanisms.
- The study’s results have been promising in lab experiments and animal models, although further research is necessary to determine the safety and effectiveness in human patients. While cautioning that the current stage is far from a ready-to-use drug, experts acknowledge the exciting potential of this approach to flip the switch on cancer cells and compel them to self-destruct.
- This innovation could potentially revolutionize cancer treatment by providing a highly specific and targeted approach to address aggressive cancers. Despite being in the early stages, this discovery holds tremendous promise and opens a new avenue for future drug development in the fight against cancer.
DEEP DIVE

This is an Executive Summary of the article “Flipping a Switch and Making Cancers Self-Destruct”, written by Gina Kolata and published by the New York Times.
Click here to access the original article.
REFERENCE PAPER

Rewiring cancer drivers to activate apoptosis
Sai Gourisankar, Andrey Krokhotin, Wenzhi Ji, Xiaofan Liu, Chiung-Ying Chang, Sauel H. Kim, Zhengnian Li, Wendy Wenderski, Juste M. Simanauskaite, Haopeng Yang, Hannes Vogel, Tinghu Zhang, Michael R. Green, Nathanael S. Gray & Gerald R. Crabtree