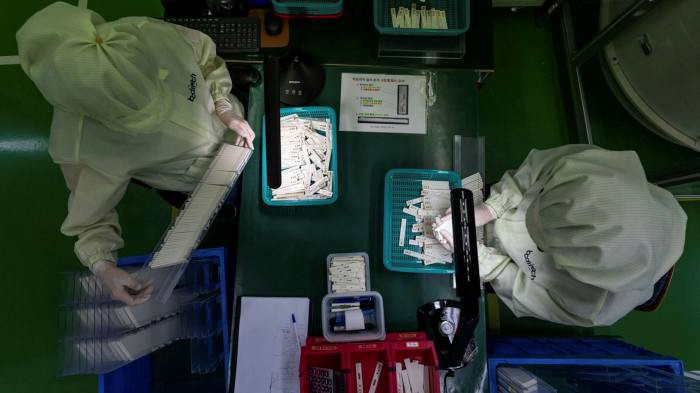
The industry is struggling to cope with fluctuating demand, and expects to outlive the pandemic, impacting the PCR segment.
This is a synthesis of the article “Covid rapid test makers race to meet overwhelming global demand”, originally published at the Financial Times, edited by the author of the blog.
SmartHealth Blog
Joaquim Cardoso MSc. (Chief Editor)
January 22, 2022
Synthesis:
What are the demand issues? And demand planning?
- Demand has “exploded” since Omicron.
- The UK has pledged to triple its extensive testing programme, while the US is requiring insurers to pay for eight tests a month, while funding free tests for the first time.
- All over the world, anxious customers have been queueing for the tests that will enable them to see loved ones, attend school or go to work.
What are the supply issues? And supply planning?
- The industry was not ready for such a huge spike in demand
- Manufacturing capacity can’t be scaled up overnight.
- The supply chain struggled to cope as rising Covid caseloads drove up demand for tests, with the problems exacerbated by governments failing to plan far enough in advance.
What are the regulatory issues?
- In the US, the Food and Drug Administration has been criticised by the industry for not moving quickly enough to approve more manufacturers.
What are the market issues?
- Many manufacturers have continued to expand capacity, adding new production lines or running existing ones 24/7 and sourcing new suppliers.
- Some companies have cautious about investing in capital projects until the US government stepped in as a big purchaser late last year.
What is the impact in the industry?
- The industry, previously best known for pregnancy tests, has transformed itself during the pandemic.
- The Covid rapid testing industry is racing to help thwart the spread of the Omicron variant, with manufacturers expanding production, hiring staff and splashing out on whole planes to send out millions of kits at a time.
What is the impact in prices?
- In European countries such as Denmark, Germany and the UK, prices for tests supplied to governments and businesses slumped after a springtime surge in infections receded last year
- Suppliers have had to scale up while paying more for components
What is expected during the pandemic, and after it?
- Abbott, the largest US provider of rapid tests which had to reboot manufacturing lines that it shut when vaccines were rolled out, is urging the US government to help maintain testing capacity during periods of low demand so it can respond to future variants and services.
- Governments and large purchasers like employers should encourage manufacturers to invest for the long term by stockpiling for future waves.
- Even when Covid-19 becomes endemic, tests will still be needed to control localised outbreaks.
- Some advocates hope that the dramatic expansion of at-home testing will outlive the pandemic, with diagnostics being used to help distinguish between Covid, flu and a common cold, or to identify other illnesses.
- Increased demand from developing countries, where PCR tests dominate despite rapid tests being cheaper, is also expected to rise
ORIGINAL PUBLICATION

Covid rapid test makers race to meet overwhelming global demand
Financial Times
January 21, 2022
The Covid rapid testing industry is racing to help thwart the spread of the Omicron variant, with manufacturers expanding production, hiring staff and splashing out on whole planes to send out millions of kits at a time.
Yet all over the world, anxious customers have been queueing for the tests that will enable them to see loved ones, attend school or go to work.
French parents have been forced to wait for hours at pharmacies, UK websites to order tests crashed while Brazilians have been warned there is a “real risk” of a shortage of tests.
The industry, previously best known for pregnancy tests, has transformed itself during the pandemic.
But the supply chain struggled to cope as rising Covid caseloads drove up demand for tests, with the problems exacerbated by governments failing to plan far enough in advance.
“All the governments suddenly want the same tests at the same time,” said Thomas Schinecker, Roche Diagnostics chief executive. “It’s not like ordering a pizza when it comes 30 minutes later. We need a bit of pre-warning.”
He said the company is in discussions with scores of governments about supplying both rapid antigen tests and the more accurate PCR tests, which require laboratory processing.
The UK has pledged to triple its extensive testing programme, while the US is requiring insurers to pay for eight tests a month, while funding free tests for the first time.
The industry was not ready for such a huge spike in demand.
William Morice, president of the American Clinical Laboratory Association, said such fluctuations make it difficult for manufacturers to sustain peak production.
“We didn’t have a lot of the advanced contracting that they had on the vaccine side, which would have helped,” he said, pointing to the lack of procurement from governments.
Abbott, the largest US provider of rapid tests which had to reboot manufacturing lines that it shut when vaccines were rolled out, is urging the US government to help maintain testing capacity during periods of low demand so it can respond to future variants and services.
In European countries such as Denmark, Germany and the UK, prices for tests supplied to governments and businesses slumped after a springtime surge in infections receded last year, according to a former executive at US-based Innova, which supplies tests to 21 countries.
“There was a lot of stock being held by distributors . . . so prices came down dramatically,” he said. Now they have ticked up again as demand has increased, the executive added.
But many manufacturers have continued to expand capacity, adding new production lines or running existing ones 24/7 and sourcing new suppliers.
They have had to scale up while paying more for components: shortages of swabs and membranes/test strips led to price increases of 25 to 40 per cent, according to a survey by FindDX, a non-governmental organisation that campaigns for greater access to diagnostic testing.
Some advocates hope that the dramatic expansion of at-home testing will outlive the pandemic, with diagnostics being used to help distinguish between Covid, flu and a common cold, or to identify other illnesses.
Some advocates hope that the dramatic expansion of at-home testing will outlive the pandemic, with diagnostics being used to help distinguish between Covid, flu and a common cold, or to identify other illnesses.
Christoph Pedain, head of the point of care business for Siemens Healthineers, said even the Biden administration’s recent order of 500m tests would have been about a quarter of a year’s demand for LFTs before the pandemic.
“The sheer magnitude is really difficult for the industry to manage,” he said. “You wouldn’t believe all the things going on behind the scenes. It starts with getting the right type of wood pulp, the fibres that form part of the paper, and goes all the way to transportation.”

Omicron has hit staffing at logistics companies. Carlos Eduardo Gouvêa, executive president of the Brazilian Association of Diagnostic Medicine, said many flights booked to import tests have been cancelled or postponed.
Pedain said prices for air freight have gone up by three or four times.
ACON, a Chinese manufacturer that produces tests for FlowFlex, one of the main brands used in the UK, said it is sending out a flight almost every day containing 2m-3m tests.
Kim Yong-kook, a director at Seegene, a molecular diagnostics company in Seoul, said it had increased its capacity by 18-fold over the past two years but demand has “exploded” since Omicron.
“We have mobilised special chartered flights twice last month, one to Europe and the other to Israel, to urgently deliver test kits that can test a total of 4m people,” he said.
Rapid tests are cheap and easier to make than PCR kits. But they are tightly regulated and complicated to manufacture:
what appears like a piece of paper within the device is actually a special nitrocellulose membrane.
It is made in a bespoke machine that can take two years to build and nine months longer to validate its consistency.
One maker of this membrane told the FT all it can do to increase capacity in the short term is try to reduce its error rate.
In the US, the Food and Drug Administration has been criticised by the industry for not moving quickly enough to approve more manufacturers.
Scott Gleason, interim chief financial officer at Pennsylvania-based rapid test maker Orasure, said the company had been cautious about investing in capital projects until the US government stepped in as a big purchaser late last year.
But Zhang Jialin, a healthcare analyst with Nomura, said fluctuating demand had made Chinese test kit makers cautious about over-investing in new capacity.
Prashant Yadav, a supply chain expert at Washington-based Centre for Global Development, said testing demand may always be “feast or famine” but planning could improve. The US did not begin procurement of key testing components until the autumn. “What on earth made us wait until October 2021?” he asked.
Yadav said governments and large purchasers like employers should encourage manufacturers to invest for the long term by stockpiling for future waves.
Even when Covid-19 becomes endemic, tests will still be needed to control localised outbreaks.
Increased demand from developing countries, where PCR tests dominate despite rapid tests being cheaper, is also expected to rise, said Emma Hannay, chief access officer at FINDDX.
Increased demand from developing countries, where PCR tests dominate despite rapid tests being cheaper, is also expected to rise
“Manufacturing capacity can’t be scaled up overnight. It can’t go from mothballed to full production in weeks,” she said. “We need a glut at times to avoid periods of shortage.”
“Manufacturing capacity can’t be scaled up overnight. It can’t go from mothballed to full production in weeks,”
Originally published at https://www.ft.com on January 21, 2022.
Names cited
Thomas Schinecker, Roche Diagnostics chief executive.
William Morice, president of the American Clinical Laboratory Association,
Christoph Pedain, head of the point of care business for Siemens Healthineers
Carlos Eduardo Gouvêa, executive president of the Brazilian Association of Diagnostic Medicine
Kim Yong-kook, a director at Seegene
Scott Gleason, interim chief financial officer at Pennsylvania-based rapid test maker Orasure
But Zhang Jialin, a healthcare analyst with Nomura,
Prashant Yadav, a supply chain expert at Washington-based Centre for Global Development
Emma Hannay, chief access officer at FINDDX.