Health Transformation Institute (HTI)
Joaquim Cardoso MSc*
Chief Researcher and Editor
November 8, 2022
*MSc from London Business School — MIT Sloan Masters Program
Key Messages:
- Traditional centralized clinical trial models have disjointed processes, disconnected technologies and redundant oversight, leading to limited participation, inefficient execution and poor retention.
- Decentralized clinical trials (DCTs) seek to solve these issues by allowing clinical trials to reach any patient or any provider anywhere while accelerating trial timelines and improving retention.
- Remote monitoring is most frequently used and effectively implemented technology, study says
- In an examination of worldwide trials, researchers noted a 50% increase in global drug trials with a DCT or virtual element from 2020 to 2021 and a 28% increase from 2021 to 2022.
- Various challenges to the use of DCTs have been cited including data standards, country-to-country regulations, and the need for clarity in regulatory guidance.
- In evaluating the costs of DCTs compared to traditional trials (Table 5), 28 (of 52 respondents) indicated that DCTs are more expensive, while 17 felt they are about the same and 7 reported they are less expensive.
Infographic
ORIGINAL PUBLICATION (full version)
The Rise of Decentralized Clinical Trials Has Led to Increased Demand For Remote Patient Monitoring Solutions
AI Doc
November 2022
Remote monitoring is here to stay.
That’s the conclusion of a survey conducted by the Tufts Center for the Study of Drug Development (CSDD).
A majority of respondents indicated that remote monitoring has impacted their organizations as a result of the pandemic and would remain the predominant approach in the future.
The survey is part of a CSDD study that assessed how approaches to decentralized and hybrid trials disrupt sponsor-CRO relationships.
It was launched in February 2022 to a diverse group of clinical research professionals and in coordination with DTRA (Decentralized Trials and Research Alliance, dtra.org) and Applied Clinical Trials.
Traditional centralized clinical trial models have disjointed processes, disconnected technologies and redundant oversight, leading to limited participation, inefficient execution and poor retention.
Traditional centralized clinical trial models have disjointed processes, disconnected technologies and redundant oversight, leading to limited participation, inefficient execution and poor retention.
Decentralized clinical trials (DCTs) seek to solve these issues by allowing clinical trials to reach any patient or any provider anywhere while accelerating trial timelines and improving retention.
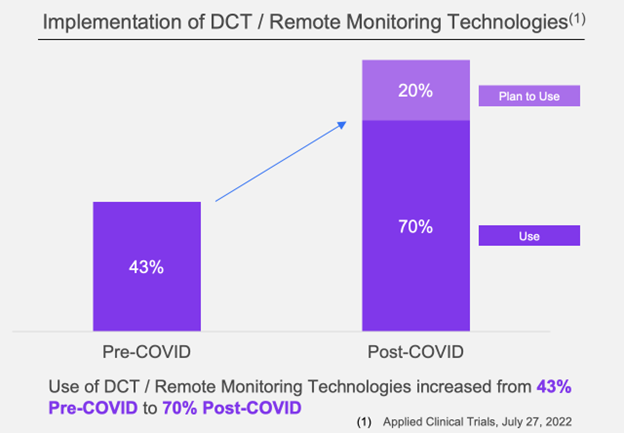
The following graph shows the growth of DCTs from pre-Covid to post-COVID.

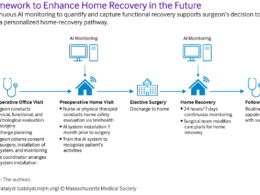
Remote monitoring is an evaluation carried out by sponsor personnel or representatives at a location other than the sites at which the clinical investigation is being conducted.
Remote monitoring is an evaluation carried out by sponsor personnel or representatives at a location other than the sites at which the clinical investigation is being conducted.
Remote monitoring, ePRO and eConsent were the decentralized clinical trial technologies used by the greatest number of respondent organizations.
The DCT technologies most frequently reported as effectively implemented were ePRO, eCOA, and remote monitoring.
Remote monitoring, ePRO and eConsent were the decentralized clinical trial technologies used by the greatest number of respondent organizations.
The DCT technologies most frequently reported as effectively implemented were ePRO, eCOA, and remote monitoring.
REFERENCE PUBLICATION (full vesrion)

The Impact of Decentralized and Hybrid Trials on Sponsor and CRO Collaborations
Applied Clinical Trials
Mary Jo Lamberti, PhD, Zachary Smith, Abigail Dirks, Tina Caruana, Trinette Mitchell, Kenneth A. Getz
July 27, 2022
Tufts CSDD study in collaboration with ten biopharmaceutical organizations and CROs examines sponsor/CRO relationships.
Decentralized clinical trials (DCTs) have accelerated in response to the pandemic and continue to evolve.
As a result, sponsor companies and contract research organizations (CROs) working in collaboration have been re-evaluating their relationships, especially in terms of structure, costs, and effectiveness.
Much of the research examining DCTs and hybrid trials has tracked increases in usage but has not specifically addressed the impact on sponsor-CRO relationships.
In an examination of worldwide trials, researchers noted a 50% increase in global drug trials with a DCT or virtual element from 2020 to 2021 and a 28% increase from 2021 to 2022. 1
Other surveys have noted growth in the implementation of DCTs, but greater usage projected for three years out. 2
Research conducted with pharmaceutical industry professionals on the use of DCTs and hybrid elements after the COVID pandemic found that in a survey of 245 investigators, 104 investigators in the U.S. indicated that
- telemedicine (74%),
- remote patient monitoring (77%), and
- remote site-initiation visits (63%) would increase. 3
Other studies report increasing use of electronic health records or prevalence and use of DCTs in numerous therapeutic areas. 4,5
Various challenges to the use of DCTs have been cited including data standards, country-to-country regulations, and the need for clarity in regulatory guidance.
Data privacy issues are also notable considerations.
Other hurdles are seen in data quality for apps, wearables and ePRO which require clinical and technical validation.
Another barrier is patients’ lack of familiarity with technology which can hinder implementation. 2,3,6
Pricing models, approaches to the implementation of DCTs, and management of vendors impact sponsor-CRO partnerships.
Research has been sparse on this topic, with a few published articles addressing the critical need for partnering with the right CROs and vendors. 2,7
In addition, the need for novel partnerships to streamline processes and improve upon data sharing have been cited. 7
Others have noted the need for multi-stakeholder collaboration across the biopharmaceutical industry to promote best practices and drive adoption. 8
As sponsor-CRO partnerships expand their collaboration, the need grows to examine the impact of DCTs and hybrid trials.
To assess this impact and gather additional research, Tufts CSDD initiated a study in collaboration with ten biopharmaceutical organizations and CROs.
Methodology and results
See the original publication [this is an excerpted version only]
Usage and implementation of DCT and hybrid solutions and technologies
For the tables, see the original publication (this is an excertped version only)
Prior to the pandemic, the largest number of respondents reported usage of ePRO (n=35), eDiaries (n=34) and eCOA (n=33) (Table 3). Currently, remote monitoring (n=38), ePRO (n=36) and eConsent (n=35) are the DCT technologies used by the greatest number of respondent organizations. The DCT technologies most frequently reported as effectively implemented were ePRO (n=30), eCOA (n=29), and remote monitoring (n=29).
A majority (40 of 50 respondents) indicated that remote monitoring impacted their organizations and would remain the predominant approach after the pandemic (29 of 54 respondents).
The fewest respondents rated the following solutions as effectively implemented (Table 4): home health services (n=15), wearables or sensor data collection (n=9), and electronic medical record (EMR)/electronic health record (EHR) data integration (n=7).
More respondents planned to use EMR and EHR data integration (n=22), wearables or sensor data collection (n=21), direct-to-patient or home IP drug shipments (n=21), and eConsent (n=20).
A high percentage of pharmaceutical and biotechnology respondents indicated that CROs were able to manage decentralized lab work including local labs (n=47 of 54 respondents), direct-to-patient (home IP drug shipments) (n=45), and eConsent (n=45) satisfactorily (‘Very Well’ or ‘Somewhat Well’).
In implementing DCT and hybrid trials with CROs and vendors, major or moderate barriers commonly identified were country challenges (n= 49 of 54 respondents); different site capabilities (n=45); and data collection challenges including quality, integrity, oversight, and ownership (n=45).
Other obstacles included resistance to change (n=44) and managing multiple vendors (n=43).
In evaluating the costs of DCTs compared to traditional trials (Table 5), 28 (of 52 respondents) indicated that DCTs are more expensive, while 17 felt they are about the same and 7 reported they are less expensive.
Asked about the future of DCTs, 23 respondents (of 51) indicated they expected the cost to increase, 13 expected the cost to remain about the same, and 15 expected the cost to decrease.
In evaluating the costs of DCTs compared to traditional trials (Table 5), 28 (of 52 respondents) indicated that DCTs are more expensive, while 17 felt they are about the same and 7 reported they are less expensive.
A comparison of the top five outsourced expenses in the time period from before the pandemic to the top five outsourced expenses in early 2020 (Table 6) yielded the same expenses, although remote monitoring moved up from fifth to second most expensive in the ranking in early 2020.
Pricing models reported by respondents before and after COVID appear to be mixed with fixed unit pricing (i.e., pricing based on completion of tasks) (26 before COVID and 23 respondents currently using) and fixed pricing (i.e., pricing that guarantees a fixed budget) at the study level (25 before COVID and 21 respondents currently using) as most prevalent. Fewer respondents indicated using incentive-based pricing or fixed pricing at the portfolio level.
A majority of pharmaceutical and biotech respondents (n=44) gave favorable ratings of CROs, indicating CROs were either somewhat or very proactive in DCT adoption (Table 7). Only 18 respondents indicated that CROs were not proactive. Fifty respondents perceived that DCTs or hybrid trials had a moderate or significant positive influence on sponsor-CRO collaborations, while 12 believed they had a moderate negative influence.

Key performance indicators
Similarities emerged among the key performance indicators (KPIs) used to evaluate sponsor-CRO relationship effectiveness and vendors implementing DCT solutions.
The top performance metrics for sponsor-CRO relationships were patient compliance, study startup, first patient first visit, enrollment, and last patient last visit to data base lock.
For DCT vendors, the top performance metrics were enrollment, final actual contract as a percent of initial contract value, first patient first visit, last patient last visit to database lock, study startup, and study startup to last patient last visit.
Training responsibilities were also consistent for sponsors and CROs/vendors with approximately three-quarters of respondents noting that sponsors and CROs/vendors were responsible for training site staff, study teams, or both.
There were no differences found among respondents by company size. The results also showed preferences for online and virtual approaches (n=60) as well as written documents and manuals (n=42) over in-person sessions (n=29).

Future outlook
In examining perceptions about outsourcing DCTs and hybrid trials over the next three to five years, 38 (of 51 respondents) indicated that outsourcing would likely increase due to advanced technologies (Table 8).
Seven indicated usage would remain the same and four felt it would likely decrease due to complications and challenges. Similarly, 44 respondents (of 52) anticipated the use of CROs and vendors that implement DCT solutions will increase as more solutions become available.
Only four believed usage would likely decrease as companies consolidate, and three felt that outsourcing would remain the same.
In examining perceptions about outsourcing DCTs and hybrid trials over the next three to five years, 38 (of 51 respondents) indicated that outsourcing would likely increase due to advanced technologies (Table 8).
Respondents offered varied opinions about the role of the CRA in the future.
Nearly half of respondents (24 of 51) indicated that CRA roles will increase with remote work, while 20 felt that the numbers would decrease.
Of those indicating a decrease, 14 felt more technological expertise and skills will be required and 6 perceived travel benefits would be curtailed.
Few respondents reported increased CRA turnover due to burnout and additional time on the job, or that the role would remain unchanged.

Implications
Although remote monitoring, ePRO and eConsent were the DCT technologies currently used by the greatest number of respondents, the results also indicated that only slightly more than half of those responding rated ePRO, eCOA and remote monitoring as effectively implemented by their organizations.
This finding may reflect challenges with implementation or investments required early on in the study.
Published research indicates that assessing ePRO technology to ensure its usability and acceptability for patients is critical. 9
Further, eCOA has numerous logistical considerations and may require added time and funding upfront, especially in terms of determining scientific requirements and qualifying suitable vendors. 10
Looking at the same DCT technologies, a surprisingly small number of respondents indicated that they planned to use the technologies in the future.
While the current study shows that fewer than half of respondents planned to use any individual technology in the future, surveys conducted during the pandemic indicated that the vast majority of respondents expected to use these technologies in future studies. 11
It may be that the general excitement surrounding these technologies is waning as organizations encounter the challenges of implementation and costs.
Relatively small numbers of respondents rated their company’s implementation of home health services as effective.
Staffing shortages due to the pandemic have impacted these services and wide variation in costs could affect usage.
Respondents’ ratings of the effectiveness of telemedicine were also fairly low relative to other technologies assessed.
This finding could perhaps be due to decreasing usage with easing COVID restrictions.
There could, however, be other factors that hinder efficiencies such as requirements of swift reporting of adverse events, necessary documentation and follow up. 12
The primary barriers reported with implementation of DCT and hybrid trials by CROs and vendors include country challenges, varied site capabilities with respect to novel technologies, and data collection.
These challenges are consistent with published data on this topic (2,3,6). Resistance to change and managing multiple vendors were also among the top five implementation hurdles.
The latter are expected given that much of the technology is novel, and the disruption brought about by a multitude of innovative solutions, especially at investigative sites could be staggering.
In addition, the number of CROs and vendors which companies must manage has increased substantially.
With the expansion of solutions globally and variation in country regulations, there are additional considerations such as data privacy and security, lack of regulatory frameworks and harmonization, and legal policies that can hinder implementation.
Use of multiple data sources can also challenge the integrity of data and clarity regarding ownership of data.
The majority of biopharma respondents rated CROs favorably for maintaining a proactive role in DCT adoption.
In addition, respondents indicated that DCTs or hybrid trials had a significant or moderate positive influence on sponsor-CRO collaborations.
Although viewed as currently more expensive with anticipated cost increases, the majority of respondents indicated that both outsourcing of these trials will increase as well as the use of CROs and vendors that implement such solutions.
Follow up research can potentially identify specific examples of relationship models due to disruptions by hybrid and decentralized trials.
An in-depth study of use cases across organizations would be invaluable and could clarify ways in which these sponsor-CRO partnerships continue to transform.
Definitions provided in the survey
Decentralized clinical trials use technologies and other solutions to create options for participation outside of conventional clinical settings, improving access and convenience.
Hybrid trials use a mix of elements from traditional clinical trials and decentralized clinical trials Remote monitoring is conducted off-site.
A remote evaluation that is carried out by sponsor personnel or representatives at a location other than the sites at which the clinical investigation is being conducted.
References
See the original publication.
Originally published at https://www.appliedclinicaltrialsonline.com on July 27, 2022.
Names mentioned:
Tufts Center for the Study of Drug Development (CSDD).