thehealthtransformation
.foundation
Joaquim Cardoso MSc
February 5, 2024
This summary is based on the article “Moderna cancer vaccine trial begins in UK, scientists hopeful of ‘dawn of a new age of treatments'”, published by The Economic Times on February 5, 2024.
What is the message?
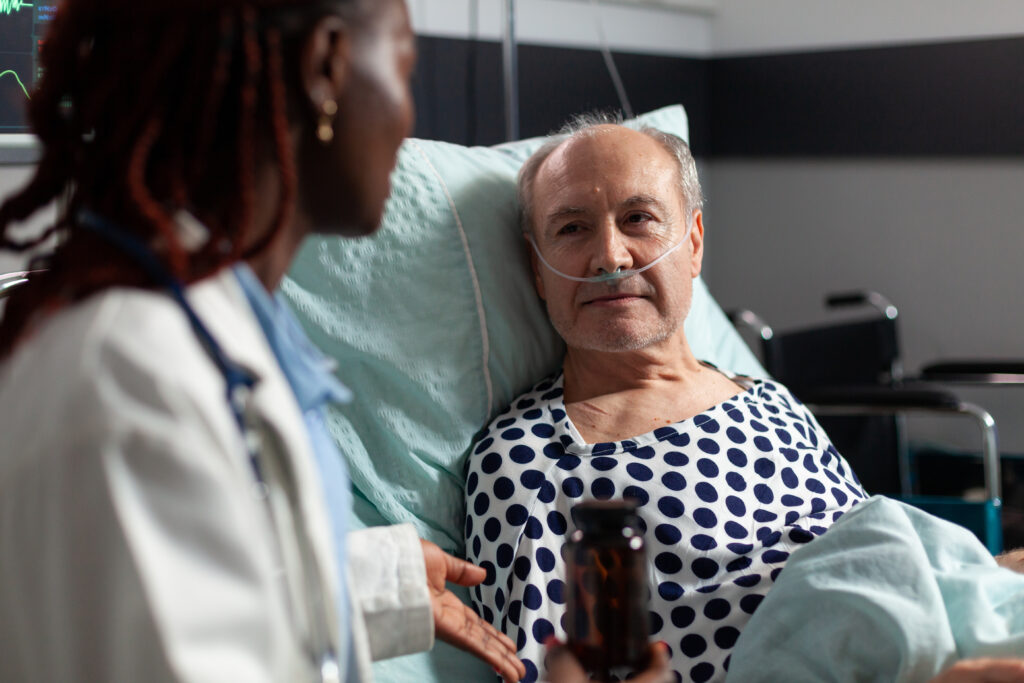
Cancer patients in the UK are part of a groundbreaking global trial for mRNA therapy, mRNA-4359, sponsored by Moderna, aiming to assess its safety and efficacy in treating melanoma, lung cancer, and other solid tumor cancers.
This therapy utilizes mRNA to train the immune system to recognize and combat cancer cells, representing a promising frontier in cancer immunotherapy.
ONE PAGE SUMMARY
What are the key points?
Global Trial: Cancer patients in the UK, starting with an 81-year-old man with treatment-resistant malignant melanoma, are participating in a global phase 1/2 clinical trial for mRNA-4359 sponsored by Moderna.
Immunotherapy Approach: mRNA-4359 employs messenger RNA (mRNA) to train the immune system by presenting common tumor markers, potentially enhancing the body’s ability to identify and eliminate cancer cells.
Collaboration and Innovation: The trial, conducted in collaboration with Moderna-UK Strategic Partnership, aims to bring mRNA vaccine manufacturing to the UK, enhancing preparedness for health emergencies and showcasing a 10-year partnership between Moderna and the UK government.
Safety Evaluation: The primary focus of the trial is to evaluate the safety and tolerance of the mRNA therapy, administered alone or in combination with the existing cancer drug pembrolizumab, an immune checkpoint inhibitor.
Patient Participation: The first patient in the UK receiving mRNA-4359 had treatment-resistant malignant melanoma, reflecting the collaboration between the UK Government and pharmaceutical companies to develop mRNA-based immunotherapies for cancer.
Trial Details: The non-randomized, open-label Phase 1/2 study involves all participants receiving the same treatment, promoting transparency and collaboration between clinicians and patients.
Promise of Cancer Vaccines: mRNA-4359 represents a promising frontier in cancer immunotherapy, falling under therapeutic cancer immunotherapies tailored to specific cancer types.
Early Results Excitement: Dr. Kyle Holen of Moderna expressed excitement about early results, envisioning a new age of cancer treatments through mRNA-based therapies.
Mobilize Trial: Conducted by Imperial College London and Imperial College Healthcare NHS Trust, the Mobilize trial at the National Institute for Health and Care Research explores mRNA-4359 as a potential treatment option for challenging-to-treat cancers.
Future Implications: Researchers believe mRNA-based cancer immunotherapies could revolutionize cancer treatments, offering a less toxic and more precise approach to therapy.
What are the key statistics?
The trial involves cancer patients, starting with an 81-year-old man with treatment-resistant malignant melanoma.
The Mobilize trial is conducted at the National Institute for Health and Care Research (NIHR) Imperial Clinical Research Facility at Hammersmith Hospital.
Conclusion
The global trial for mRNA-4359 offers hope for advancing cancer treatments, with early results indicating its potential to revolutionize therapy by leveraging the body’s immune system against cancer cells.
The collaboration between Moderna, the UK government, and healthcare institutions emphasizes the commitment to innovation and preparedness for future health challenges.
While in the early stages, the Mobilize trial lays essential groundwork for less toxic and more precise cancer therapies.
To read the original publication, click here.











