A Chinese antiviral pill was found to be as effective as Paxlovid in a Phase 3 trial, and it appeared to cause fewer side effects.
health transformation . institute
knowledge platform & advisory consulting
Joaquim Cardoso MSc
Chief Researcher, Editor and Senior Advisor
December 31, 2022
SOURCEs: NBC News & NEJM
NBC News
Aria Bendix
Dec. 29, 2022
A new antiviral pill for Covid was found to be as effective as Paxlovid at curbing mild to moderate illness among people at high risk of severe disease in a Phase 3 trial in China.
The results, published Wednesday in The New England Journal of Medicine, suggest that the treatment had fewer side effects than Paxlovid, the go-to antiviral for high-risk patients.
Around 67% of people who took the experimental pill, called VV116, reported side effects, compared to to 77% who took Paxlovid.
The results, published Wednesday in The New England Journal of Medicine, suggest that the treatment had fewer side effects than Paxlovid, the go-to antiviral for high-risk patients.
Around 67% of people who took the experimental pill, called VV116, reported side effects, compared to to 77% who took Paxlovid.
The new pill was also less likely than Paxlovid to cause unexpected side effects due to reactions with other medications, such as those for insomnia, seizures or high blood pressure.
“You have a medication that looks to be just as good as Paxlovid, but less cumbersome,” said Dr. Panagis Galiatsatos, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
“You have a medication that looks to be just as good as Paxlovid, but less cumbersome,”

VV116 is similar to the antiviral remdesivir, which the Food and Drug Administration has approved as an IV infusion.
But the team behind the new drug — pharma companies Junshi Biosciences and Vigonvita Life Sciences — tweaked the formula so that the body can absorb it in pill form, said Dr. Peter Gulick, an associate professor of medicine at Michigan State University.
Gilead Sciences, which developed remdesivir, is testing a similar oral version of its drug.
VV116 is similar to the antiviral remdesivir, which the Food and Drug Administration has approved as an IV infusion.
But the team behind the new drug — pharma companies Junshi Biosciences and Vigonvita Life Sciences — tweaked the formula so that the body can absorb it in pill form
Gulick said people who have received intravenous remdesivir thus far have not seen their symptoms rebound in the days or weeks following the treatment the way people have with Paxlovid.

In the trial of VV116, more than 380 people took the experimental drug, while a similarly sized group took Paxlovid. Both treatment courses lasted five days.
The median time to recovery — defined as no Covid symptoms for two consecutive days — was four days for VV116 recipients and five days for those who took Paxlovid.
After four weeks, around 98% of all participants had recovered, and no one developed severe Covid.
Study co-author Ren Zhao, a professor at Shanghai Jiao Tong University School of Medicine, called the trial a “great success” in a news release Thursday.
When it comes to specific side effects, around 26% of the trial participants who took Paxlovid said it altered their sense of taste — food tasted sour, sweet, bitter or metallic — but just 4% of people who took VV116 reported that experience.
Although some people in both groups had elevated levels of triglycerides (fat in the blood that can increase the risk of heart disease or stroke), a smaller share of those in the VV116 group saw that effect: 11% compared to 21% of participants who took Paxlovid.
That reduced likelihood of side effects is “a big deal,” Galiatsatos said.
That reduced likelihood of side effects is “a big deal,”
Three-quarters of the trial participants were vaccinated, though the study found consistent results regardless of vaccine status.
U.S. medical experts said it will be important to study the pill in a larger, more diverse group.
Such trials could better catch rare side effects and examine how the drug holds up against newer omicron subvariants that have emerged since the study period.
Galiatsatos said the FDA is likely to ask for more data before considering emergency authorization.
But he added that the pill seems promising: “It looks like we might have another tool in the toolbox.”
Antiviral drugs are designed to stop a virus from replicating.
Because they don’t spur an antibody response the way vaccines do, the effectiveness of antivirals is less sensitive to changes in the coronavirus as new variants and subvariants evolve, according to Gulick.
“This whole group of agents is going to be very important for the future,” he said.
Besides remdesivir, the FDA has granted emergency authorization to two antiviral pills: Paxlovid and molnupiravir.
The National Institutes of Health recommends Paxlovid, with molnupiravir as an alternative in situations when neither Paxlovid nor remdesivir is available or appropriate.
Paxlovid, though effective at preventing severe disease, comes with a few drawbacks.
It contains a medication called ritonavir, which can cause liver damage — mostly in patients with pre-existing liver problems — and it can have negative interactions with other drugs like statins or heart medications.
“A lot of medical providers were very hesitant in using Paxlovid in many patients because they were concerned about the drug-drug interactions,” Gulick said.
“A lot of medical providers were very hesitant in using Paxlovid in many patients because they were concerned about the drug-drug interactions,”
Many patients at the highest risk of severe Covid are on multiple drugs, he added.
“Paxlovid is still a great drug, but there’s a variety of reasons to keep it from truly reaching everyone that it needs to,” Galiatsatos said.
Experts are hopeful that VV116 could fill some of these gaps, assuming it performs well in larger studies.
Standard Phase 3 drug trials involve up to 3,000 participants, according to the FDA. Paxlovid’s late-stage trial included more than 2,200 people.
“Rare side effects you’re only going to pick up when you launch into a bigger population,” Galiatsatos said.
“It’s like playing the lottery: 1 in 100 aren’t going to win, but one in a million will, because you increase your odds of seeing a rare event occur.”
Originally published at https://www-nbcnews-com.cdn.ampproject.org on December 29, 2022.
ORIGINAL PUBLICATION

VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19
List of authors.
NEJM
Zhujun Cao, M.D., Ph.D., Weiyi Gao, M.D., Ph.D., Hong Bao, M.D., Ph.D., Haiyan Feng, M.D., Shuya Mei, M.D., Ph.D., Peizhan Chen, Ph.D., Yueqiu Gao, M.D., Ph.D., Zhilei Cui, M.D., Ph.D., Qin Zhang, M.D., Ph.D., Xianmin Meng, Ph.D., Honglian Gui, M.D., Ph.D., Weijing Wang, M.D., Ph.D., Yimei Jiang, M.D., Zijia Song, M.D., Ph.D., Yiqing Shi, M.D., Jing Sun, M.D., Ph.D., Yifei Zhang, M.D., Ph.D., Qing Xie, M.D., Ph.D., Yiping Xu, Ph.D., Guang Ning, M.D., Yuan Gao, M.D., Ph.D., and Ren Zhao, M.D., Ph.D.
December 28, 2022
Abstract
BACKGROUND
- Nirmatrelvir–ritonavir has been authorized for emergency use by many countries for the treatment of coronavirus disease 2019 (Covid-19).
- However, the supply falls short of the global demand, which creates a need for more options.
- VV116 is an oral antiviral agent with potent activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
METHODS
- We conducted a phase 3, noninferiority, observer-blinded, randomized trial during the outbreak caused by the B.1.1.529 (omicron) variant of SARS-CoV-2.
- Symptomatic adults with mild-to-moderate Covid-19 with a high risk of progression were assigned to receive a 5-day course of either VV116 or nirmatrelvir–ritonavir.
- The primary end point was the time to sustained clinical recovery through day 28. Sustained clinical recovery was defined as the alleviation of all Covid-19–related target symptoms to a total score of 0 or 1 for the sum of each symptom (on a scale from 0 to 3, with higher scores indicating greater severity; total scores on the 11-item scale range from 0 to 33) for 2 consecutive days.
- A lower boundary of the two-sided 95% confidence interval for the hazard ratio of more than 0.8 was considered to indicate noninferiority (with a hazard ratio of >1 indicating a shorter time to sustained clinical recovery with VV116 than with nirmatrelvir–ritonavir).
RESULTS
- A total of 822 participants underwent randomization, and 771 received VV116 (384 participants) or nirmatrelvir–ritonavir (387 participants).
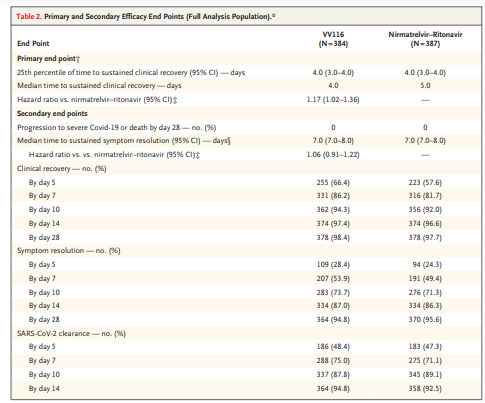
- The noninferiority of VV116 to nirmatrelvir–ritonavir with respect to the time to sustained clinical recovery was established in the primary analysis (hazard ratio, 1.17; 95% confidence interval [CI], 1.01 to 1.35) and was maintained in the final analysis (median, 4 days with VV116 and 5 days with nirmatrelvir–ritonavir; hazard ratio, 1.17; 95% CI, 1.02 to 1.36).
- In the final analysis, the time to sustained symptom resolution (score of 0 for each of the 11 Covid-19–related target symptoms for 2 consecutive days) and to a first negative SARS-CoV-2 test did not differ substantially between the two groups.
- No participants in either group had died or had had progression to severe Covid-19 by day 28.
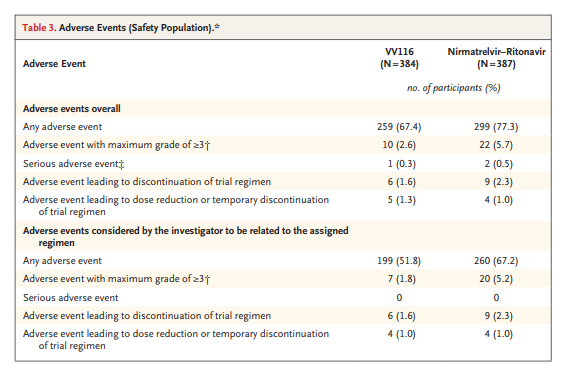
- The incidence of adverse events was lower in the VV116 group than in the nirmatrelvir–ritonavir group (67.4% vs. 77.3%).
CONCLUSIONS
- Among adults with mild-to-moderate Covid-19 who were at risk for progression, VV116 was noninferior to nirmatrelvir–ritonavir with respect to the time to sustained clinical recovery, with fewer safety concerns. (Funded by Vigonvita Life Sciences and others; ClinicalTrials.gov number, NCT05341609. opens in new tab; Chinese Clinical Trial Registry number, ChiCTR2200057856.)
Among adults with mild-to-moderate Covid-19 who were at risk for progression, VV116 was noninferior to nirmatrelvir–ritonavir with respect to the time to sustained clinical recovery, with fewer safety concerns.

Introduction
The coronavirus disease 2019 (Covid-19) pandemic continues to spread rapidly worldwide,1,2 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved into variants with increasing transmissibility and capability of evading human immunity (e.g., the B.1.1.529 [omicron] variant).3–5 A widespread and timely distribution of efficacious antiviral therapy is an important part of the response.6,7
Currently, nirmatrelvir–ritonavir8 is recommended by World Health Organization (WHO) guideline for treating mild-to-moderate Covid-19.9 Nirmatrelvir is an oral inhibitor of the SARS-CoV-2 3-chymotrypsin–like cysteine protease enzyme that can be dispensed at community pharmacies and has been authorized for emergency use by many countries. However, access to nirmatrelvir is limited worldwide, and its effectiveness depends on ritonavir,10 which has multiple drug–drug interactions warranting specialized assessment before prescription. Remdesivir is also recommended11 but needs to be administered intravenously, which limits its widespread use during the pandemic. Therefore, several oral analogues of remdesivir have been developed to address this issue, including GS-621763,12 ATV006,13 and VV116.14,15
VV116 is a deuterated remdesivir hydrobromide with oral bioavailability and potent activity against SARS-CoV-2 in studies in animals15 and satisfactory safety and side-effect profiles in phase 1 trials.16 A preliminary small-scale study has shown a shorter viral shedding time in patients with Covid-19 who received VV116 within 5 days after the first positive test than in those who received regular care.17 However, the efficacy of VV116 for clinical recovery, symptom resolution, and prevention of disease progression remains unknown, particularly as compared with nirmatrelvir–ritonavir. In addition, the safety profiles of VV116 have not been fully assessed. Here, we report the results of a phase 3 trial of VV116 as compared with nirmatrelvir–ritonavir for oral treatment of symptomatic participants at high risk for progression to severe Covid-19 during the omicron outbreak.
Methods & other sections
See the original publication (this is an excerpt version)
Discussion
In light of the preliminary positive findings of a reduction in viral shedding time among patients with SARS-CoV-2 infection who were taking VV116,17 the current trial compared VV116 with nirmatrelvir–ritonavir to assess clinical end points and adverse events. This trial showed that in symptomatic adults hospitalized with mild-to-moderate Covid-19 who were at high risk for severe disease, a 5-day course of oral treatment with VV116 was noninferior to nirmatrelvir–ritonavir in shortening the time to sustained clinical recovery. This noninferiority in efficacy was seen in the full analysis population, the per-protocol population, and in participants who started treatment within 5 days after symptom onset. The point estimates of secondary end points also suggested that VV116 was better than or similar to nirmatrelvir–ritonavir with respect to the time to sustained symptom resolution and to a first negative SARS-CoV-2 test. No participants in either group died or had progression to severe or critical Covid-19. Participants in the VV116 group had a lower incidence of adverse events than those in the nirmatrelvir–ritonavir group.
The administration of oral antiviral agents is feasible early in infection. Such therapies, if given promptly, could help mitigate hospitalization burden, facilitate postexposure prophylaxis, and potentially minimize household transmission.
This trial was performed in Shanghai, China, during an outbreak of Covid-19 (March through June 2022) involving more than 600,000 infections.21 SARS-CoV-2 genomic analysis of specimens from 129 patients in this period showed the BA.2.2 sublineage in all of them,20 which suggests that the major variant involved in our trial was omicron. In this population, the median time to sustained clinical recovery or symptom resolution in both trial groups was shorter than those reported in other trials, such as those evaluating REGEN-COV (14 days)22 and bamlanivimab with or without etesevimab (8 days).23
Another feature of this trial is that 75.7% of the participants had been vaccinated against SARS-CoV-2, which reflects the current reality of population immunity; vaccinated persons have been excluded from most trials, given the rapidly changing landscape of the Covid-19 response.8 Therefore, we prespecified and conducted subgroup analyses according to vaccination status. The results were similar in participants with previous vaccination and those without previous vaccination. Recent studies have shown that treatment with nirmatrelvir–ritonavir in vaccinated patients with Covid-19 is associated with a reduced risk of hospitalization or progression to severe Covid-19, as well.24–26
In this trial, fewer adverse events occurred in the VV116 group than in the nirmatrelvir–ritonavir group. Unlike nirmatrelvir–ritonavir, which has drug–drug interactions with multiple medications,9 VV116 does not inhibit or induce major drug-metabolizing enzymes or inhibit major drug transporters, so interaction with concomitant medications is less likely. Transient dysgeusia was reported in one quarter of the participants receiving nirmatrelvir–ritonavir in this trial, a proportion higher than that previously reported in the EPIC-HR (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) trial (5.6%)9; this adverse event warrants more attention in future trials. In addition, the incidence of dyslipidemia was relatively high among both nirmatrelvir–ritonavir recipients and VV116 recipients. Although this adverse reaction has been noted with long-term use of ritonavir in patients with human immunodeficiency virus infection,27 the possible effect of nirmatrelvir or VV116 on lipid metabolism needs further investigation.
The trial has several limitations. First, we were not able to conduct this trial with a double-blind and double-dummy design because the production of the placebo tablet for nirmatrelvir–ritonavir was not completed before the trial began owing to the omicron outbreak. Second, the trial involved Chinese adults infected with omicron subvariants in a single geographic area, so the results require validation in more heterogeneous populations with greater diversity of viral variants. Third, it is possible that symptoms could have recurred after 2 consecutive days without symptoms. Fourth, the WHO ordinal scale that was used to evaluate outcomes was not ideal for detecting differences among participants with mild Covid-19, especially when discharge decisions may be driven by factors other than clinical improvement. Fifth, no conclusions can be made about the efficacy of VV116 for the prevention of progression to severe or critical Covid-19 or death, because no events occurred in either group. Possible efficacy for this outcome is planned to be evaluated in a separate trial (ClinicalTrials.gov number, NCT05242042. opens in new tab). Sixth, we did not recognize SARS-CoV-2 rebound after nirmatrelvir–ritonavir treatment until the release of the Centers for Disease Control and Prevention advisory on May 24, 2022.28 Data on such rebounds were very limited and not suitable for analysis in our trial.
In this trial, early administration of oral VV116 was noninferior to nirmatrelvir–ritonavir in shortening the time to sustained clinical recovery in participants with mild-to-moderate Covid-19 who were at high risk for progression to severe disease.
VV116 also had fewer safety concerns than nirmatrelvir–ritonavir.
In this trial, early administration of oral VV116 was noninferior to nirmatrelvir–ritonavir in shortening the time to sustained clinical recovery in participants with mild-to-moderate Covid-19 who were at high risk for progression to severe disease.
VV116 also had fewer safety concerns than nirmatrelvir–ritonavir.
Author Affiliations
From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases (Y.Z., G.N.), and Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the People’s Republic of China, Shanghai Key Laboratory for Endocrine Tumors (Y.Z., G.N.), Ruijin Hospital, the Department of Critical Care Medicine, Renji Hospital (S.M., Yuan Gao), the Department of Respiratory Medicine, Xinhua Hospital (Z. Cui), and the Department of Good Clinical Practice Office and Phase I Unit, Tongren Hospital (Q.Z.), Shanghai Jiao Tong University School of Medicine, the Department of Respiratory and Critical Medicine, Shanghai Pudong Hospital, Fudan University Pudong Medical Center (H.B.), the Departments of Pain Rehabilitation (H.F.) and Pharmacology (X.M.), Shanghai Public Health Clinical Center, Fudan University, and the Department of Liver Disease, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (Yueqiu Gao) — all in Shanghai, China.
Pharma companies
Junshi Biosciences and Vigonvita Life Sciences
NAMES MENTIONED
Dr. Panagis Galiatsatos, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
pharma companies Junshi Biosciences and Vigonvita Life Sciences
Dr. Peter Gulick, an associate professor of medicine at Michigan State University.
Study co-author Ren Zhao, a professor at Shanghai Jiao Tong University School of Medicine, called the trial a “great success” in a news release Thursday.
NAMES MENTIONED
Dr. Panagis Galiatsatos, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
pharma companies Junshi Biosciences and Vigonvita Life Sciences
Dr. Peter Gulick, an associate professor of medicine at Michigan State University.
Study co-author Ren Zhao, a professor at Shanghai Jiao Tong University School of Medicine, called the trial a “great success” in a news release Thursday.