health transformation institute
vaccines & vaccination institute
Joaquim Cardoso MSc
Senior Advisor and
Chief Researcher & Editor
January 15, 2023
KEY MESSAGE(S)
- Participants who received the bivalent vaccine had lower hospitalization and mortality rates due to Covid-19 than non-recipients up to 70 days after vaccination.
- The study results demonstrate that vaccination with a bivalent booster among eligible adults aged ≥65 was associated with a statistically significant 81% reduction in Covid-19 hospitalizations.
- The bivalent booster was also associated with a borderline statistically significant 86% reduction in Covid-19 mortality, probably due to the relative scarcity of Covid-19 mortality events.
- The results show a low response rate.
DEEP DIVE
Effectiveness of the Bivalent mRNA Vaccine in Preventing Severe COVID-19 Outcomes: An Observational Cohort Study
Citation
Arbel, Ronen and Peretz, Alon and Sergienko, Ruslan and Friger, Michael and Beckenstein, Tanya and Yaron, Shlomit and Hammerman, Ariel and Bilenko, Natalya and Netzer, Doron, Effectiveness of the Bivalent mRNA Vaccine in Preventing Severe COVID-19 Outcomes: An Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=4314067 or http://dx.doi.org/10.2139/ssrn.4314067
ABSTRACT
Background:
- During Late 2022, the SARS-CoV-2 Omicron BA.5 sublineages accounted for most of the sequenced viral genomes worldwide.
- Bivalent mRNA vaccines contain an ancestral SARS-CoV-2 strain component plus an updated component of the Omicron BA.4/BA.5.
- Since September 2022, a single bivalent booster dose has been recommended for adults who have completed a primary vaccination series and are at high risk for severe Covid-19 disease.
- Evidence regarding the effectiveness of the bivalent vaccine in reducing hospitalizations and death due to Covid-19 is warranted.
Methods:
- This retrospective cohort study included all members of Clalit Health Services, aged ≥65, eligible for a bivalent booster.
- Hospitalizations and death due to Covid-19 among participants who received the bivalent vaccine were compared with those who did not.
- A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association between the bivalent vaccine and Covid-19 outcomes while adjusting for demographic factors and coexisting illnesses.
Findings:
- A total of 622,701 participants met the eligibility criteria.
- Of those, 85,314 (14%) received a bivalent-booster during the 70-day study period.
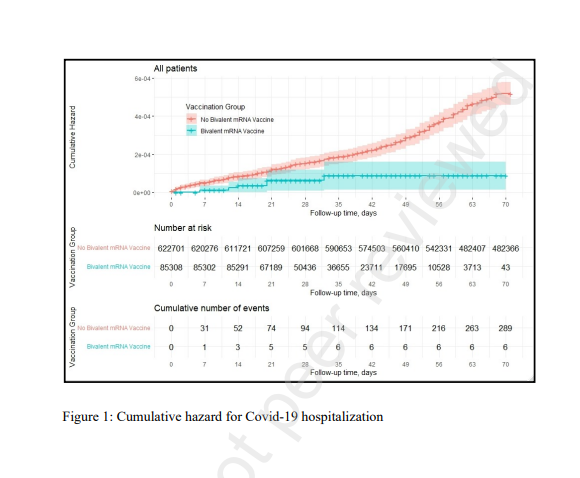
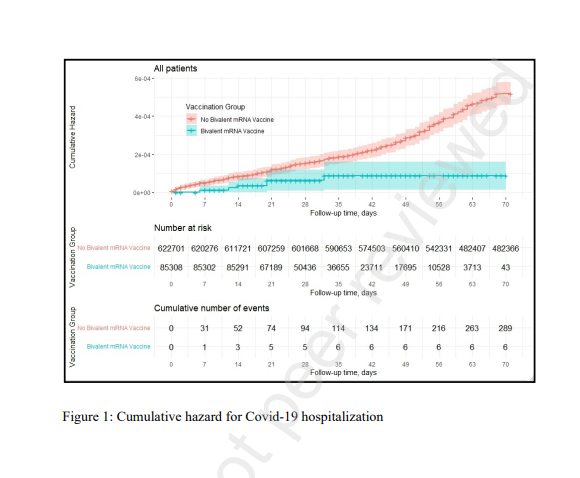
- Hospitalization due to Covid-19 occurred in 6 bivalent recipients and 297 participants who did not, adjusted hazard ratio (HR): 0.19 (95% CI, 0.08–0.43).
- Death due to Covid-19 occurred in 1 bivalent recipient and 73 participants who did not, adjusted HR 0.14: (95% CI, 0.02–1.04).
Interpretation:
- Participants who received the bivalent vaccine had lower hospitalization and mortality rates due to Covid-19 than non-recipients up to 70 days after vaccination.
Funding Information: None.
Declaration of Interests: All authors report no conflict of interest.
About the authors & affiliations
Ronen Arbel*, Ph.D.1,2 ,
Alon Peretz*, M.D.1,3,
Ruslan Sergienko, M.A.4 ,
Michael Friger, Ph.D.4 ,
anya Beckenstein, B.Sc.1 ,
Shlomit Yaron, M.D.1 ,
Ariel Hammerman, Ph.D.1
Natalya Bilenko **, M.D.4 ,
Doron Netzer**, M.D.1 .
1. Community Medical Services Division, Clalit Health Services, Tel-Aviv, Israel.
2. Maximizing Health Outcomes Research Lab, Sapir College, Sderot, Israel.
3. School of Public Health, University of Haifa, Haifa, Israel
4. Faculty of Health Sciences, Ben-Gurion University of the Negev, Beersheba, Israel
Originally published at https://papers.ssrn.com.

Discussion — Summary of Results
Our results demonstrate that vaccination with a bivalent booster among eligible adults aged ≥65 was associated with a statistically significant 81% reduction in Covid-19 hospitalizations.
The bivalent booster was also associated with a borderline statistically significant 86% reduction in Covid-19 mortality, probably due to the relative scarcity of Covid-19 mortality events.
Our results show a low response rate.