Credit for the image on top: Dr. Seth Berkley. Gavi/Tony Noel
Seth Franklin Berkley, is an American medical , the CEO of the and a global advocate of the power of vaccines. He is the founder and former president and CEO of the (IAVI).
TIME
Jeffrey Kluger
Series: A Blueprint for Preventing the Next Pandemic
June 9, 2021
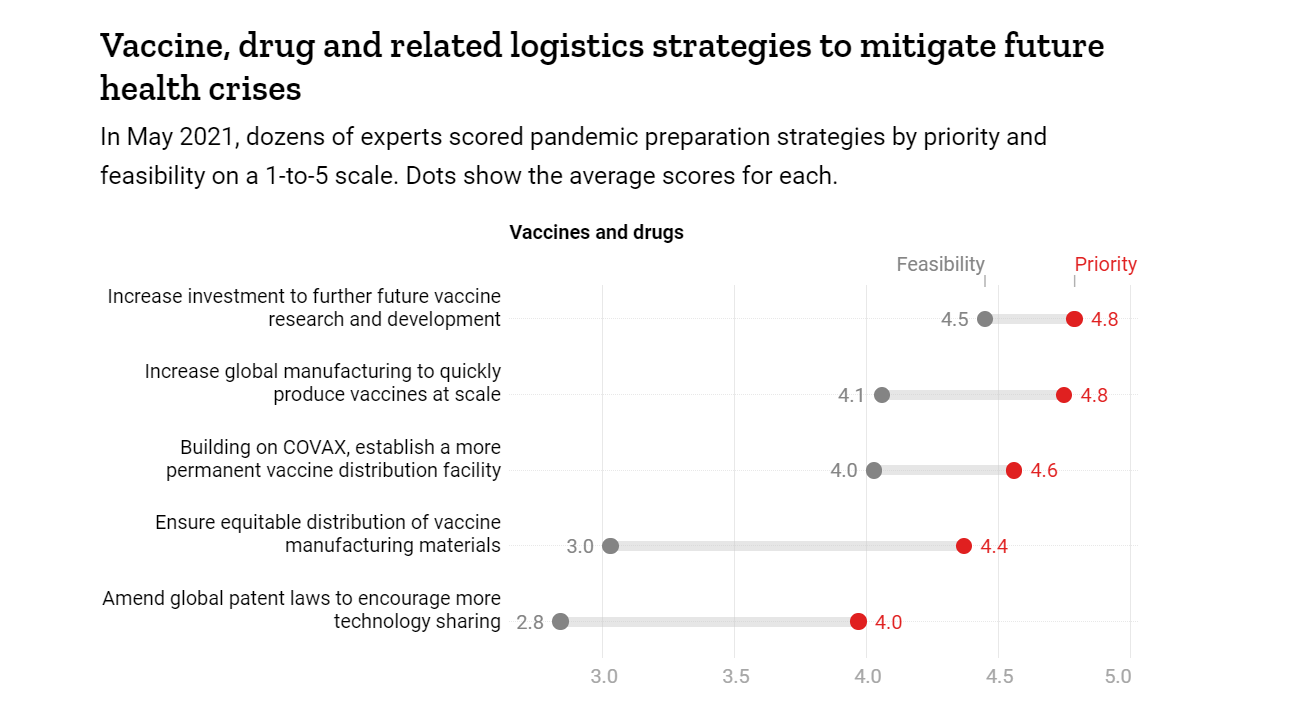
It should worry everyone that experts surveyed by TIME regarded both increasing funding in a post-COVID-19 world for vaccine development and scaling up of manufacturing capacity feasible- but improving equitable vaccine distribution was not.
To stop the next pandemic in its tracks we need to ensure that people all over the world are protected quickly, and that will entail having all these pieces in place.
The good news is, all these elements are feasible, and indeed starting to work today.
On vaccine R&D, the Coalition for Epidemic Preparedness Innovations (CEPI), was set up with the precise purpose of identifying and investing in R&D for vaccines against emerging infectious diseases with epidemic potential.
So, when it came to COVID-19, with CEPI’s and other R&D support, as well as industry engagement, the scientific and vaccine manufacturing community rallied, producing the first safe and effective vaccine in record time-just 327 days. Today we have not just one but 15 in widespread use.
Increased investment now could get us there even faster the next time, particularly given the potential of the relatively new RNA vaccine technologies that have proved so effective with COVID-19.
These plug-and-play vaccine technologies not only make it possible to
- identify and develop antigens rapidly, but
- much of the regulatory testing and approval can be done in advance, even before we know what the threat is.
Vaccine, drug and related logistics strategies to mitigate future health crises
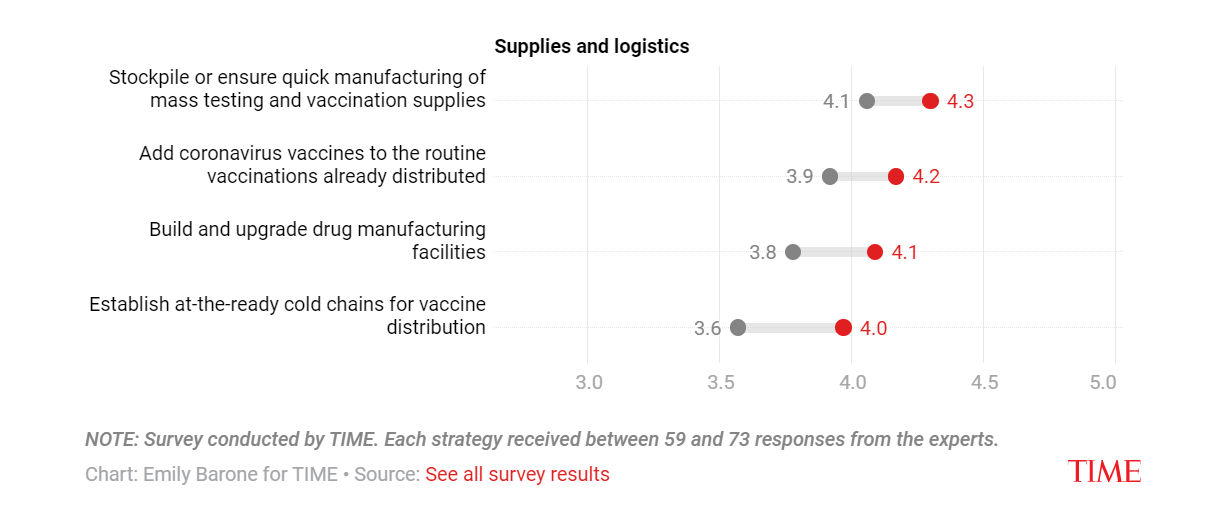
As for manufacturing, it may be difficult to immediately discern when there are severe supply shortages, but the world has actually rapidly built up manufacturing capacity during COVID-19.
Waiving intellectual property has been talked about a lot as a potential solution for boosting production.
But the growth we have seen in the past year has been achieved through technology transfers, where both the intellectual property and the vital know-how needed to make vaccines is shared between manufacturers.
However, we need to do more.
Given the extremely large number of doses needed during a pandemic, export bans of vaccines and essential components and supply bottlenecks have led to a vaccine divide.
Currently, more than a third of adults in high-income countries have now been vaccinated, while less than 1% of those in low-income countries have had their first jab.
To prevent this kind of scenario from happening the next time round and ensure that those most at risk are prioritized wherever they are, it is not distribution channels we are lacking, but global manufacturing capacity.
- We already have highly effective distribution channels, through COVAX and its partners, and
- we already have access to doses, enough to protect 1.8 billion people in lower-income economies by early next year, enough to protect almost 30% of people in these countries.
But through investments now to increase global manufacturing capacity, particularly in emerging economies, and support of technology transfers, the next time a pandemic strikes we can get there sooner.
Originally published at https://time.com on June 9, 2021.