WHO
André Ilbawi, Cherian Varghese — Executive WHO editors and writers
February 2020
Key messages
1.Governments have committed themselves to preventing and controlling cancer in several global declarations, including the 2030 United Nations Agenda for Sustainable Development. Progress in meeting their commitments has been slow.
2.WHO “best buys” for the prevention and control of NCDs should be implemented by all governments, with existing legal instruments and frameworks for an integrated response.
3.The SDGs cannot be achieved without accessible cancer management services that provide value for money. Cancer control is an integral component of the path towards UHC and a reduction in premature mortality by 2030.
4.Investment in an essential package of cancer interventions will provide a positive return on investment, with meaningful social and economic returns, including increased productivity and equity.
5.By 2030, more than 7 million cancer deaths in LMIC can be avoided; the investment required will be US$ 2.70 per person in LIC, US$ 3.95 per person in lower-middle-income countries and US$ 8.15 per person in upper-middle-income countries.
2.1 Global commitments to cancer control
Cancer is rising on the global health and development agendas.
For many years, cancer was considered a disease of wealthy countries, and the global public health emphasis was on prevention.
With recognition of the rapidly increasing global burden and that less than 50% all cancer cases are preventable with current knowledge and interventions, governments have increased the importance of comprehensive cancer control on the development agendas.
Cancer and other NCDs constitute one of the major barriers to development in the 21st century, and strong commitments have been made in United Nations and World Health assemblies during the past decade (Fig. 2.1).
Fig 2.1. International political commitments relevant to cancer control
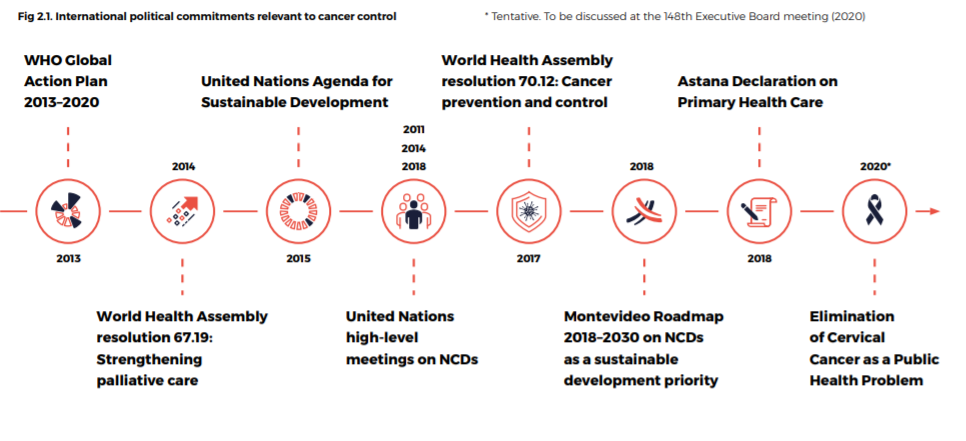
The first United Nations high-level meeting on NCDs in 2011 resulted in a political declaration and commitments by Member States (Fig. 2.1).
The next year, in 2012, WHO Member States agreed to the Global Action Plan for the Prevention and Control of NCDs 2013 -2020, which includes the goal of a 25% reduction in premature mortality from NCDs by 2025, with targets for reducing risk factors and changing health systems.
Public health commitments to a multisectoral response to NCDs are also reflected in the United Nations 2030 Agenda for Sustainable Development, with SDG 3.4 as the banner for cancer control and with recognition of the centrality of UHC, the importance of caring for children and the elderly and the necessity of palliative care.
To track progress in achieving the Global action plan, Member States adopted time-bound commitments to set national targets for NCDs, prepare national plans, reduce the risk factors for NCDs and strengthen health system responses to NCDs.
Progress in meeting these commitments has, however, been disappointing (Fig. 2.2).
Fig. 2.2. Status of 10 indicators to chart progress in national responses and fulfilment of commitments made since the first United Nations high-level meeting on NCDs in 2011
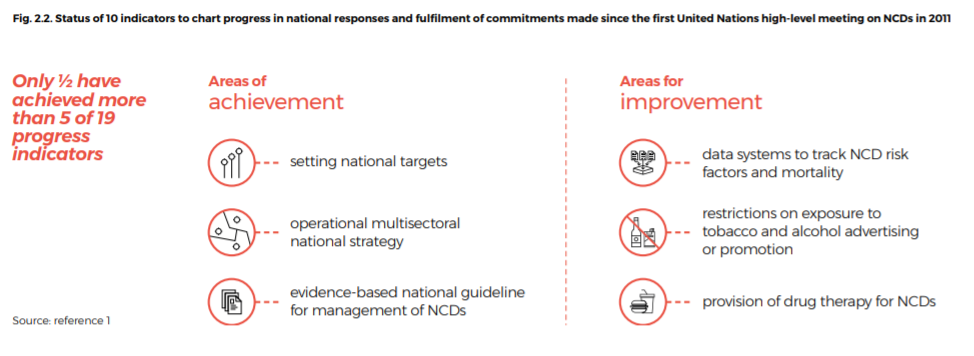
Source: reference 1[1]
With a mandate from Member States, WHO has framed the global response to cancer through an integrated approach, built on UHC and strengthening health systems through robust NCCPs[2] [3] (2,3).
This is reflected in three interconnected WHO targets:
- to ensure that one billion more people benefit from UHC,
- one billion more people enjoy better health and well-being, including protection against cancer risk factors, and
- one billion more people are better protected from health emergencies[4] (4).
WHO regional frameworks for action have also been adopted[5] [6] (5,6). Global commitments to reducing the cancer burden have been made by many other United Nations agencies, including the UN Interagency Task Force on NCDs[7] (7).
Recognizing that progress in cancer control must be accelerated and to meet its global mandate, WHO has launched two global initiatives to address the cancer burden (Box 2.1).

Box 2.1 WHO global initiatives to reduce the cancer burden
WHO remains committed to supporting governments in formulating comprehensive responses to the growing cancer burden, with other United Nations agencies and global partners.
Elimination of cervical cancer:
The initiative toward the elimination of cervical cancer as a public health problem includes reducing the age-adjusted incidence rate in every country to less than 4 per 100 000 women per year and scaling up capacity to treat and provide palliative care to women with cervical cancer.
At present, national age-standardized incidence rates per 100 000 women range from more than 70 in the countries at highest risk to less than 10 in those at lowest risk. Although the incidence cannot be reduced to 0 with current interventions, the elimination threshold is achievable in every country during this century. WHO, its Member States and partners are activating resources to assist policy-makers and other stakeholders to invest and implement policies and programmes to eliminate cervical cancer.
Global Initiative for Childhood Cancer:
The aim of this initiative is to ensure the survival of at least 60% of children with cancer by 2030, thereby saving an additional one million lives and reducing suffering for all. This represents a doubling of the present global cure rate for childhood cancer.
The Initiative includes increasing the priority of these cancers by governments and improving the capacity of countries for diagnosing, treating and monitoring the outcomes of these cancers. It involves active global partners and networks.
Within a structured “CURE All” plan, six index cancers and focused implementation in countries, the care of every child with cancer will be improved through scientific advances and assurance that children will benefit from them.
2.2 Meeting global commitments in the national context
2.2.1 Adapting commitments to regions and countries
Global commitment to international goals and targets must be translated into action nationally and regionally according to their political and legal systems, the epidemiology of cancer, risk factors and their resources and technical expertise.
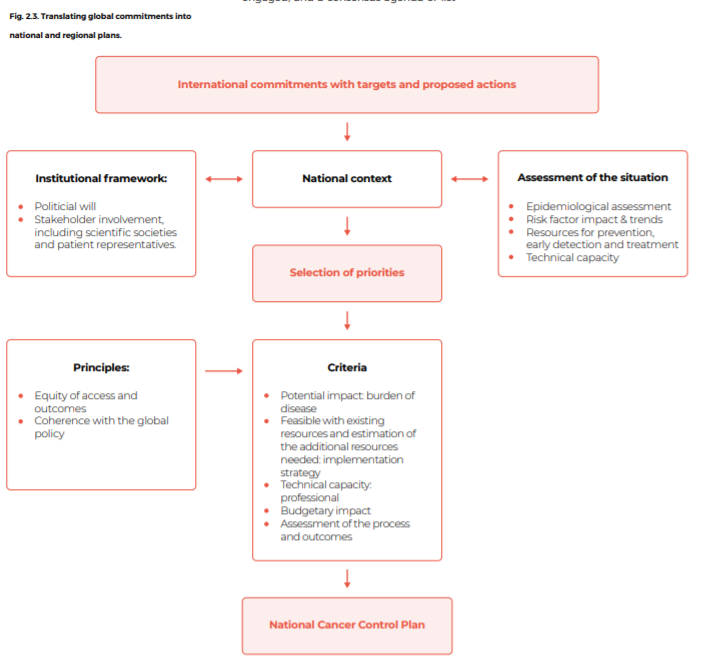
Strategic priorities should be set on the basis of accurate data and feasibility; appropriate stakeholders should be engaged; and a consensus agenda or list of priorities drawn up for an NCCP (Fig. 2.3; see also chapter 6).
The priorities should meet national and global targets.
In 2019, national targets in the NCD plans of approximately 67% of countries were aligned with global targets[8] (1).
Fig. 2.3. Translating global commitments into national and regional plans.
Regional bodies with knowledge of their territories and populations have a central role in determining regional priorities for their member States, including influencing their health policies and formulating regional responses (Box 2.2).
For example, the European Union is involved in many areas of health and development that are directly relevant to cancer. Similarly, the Union of South American Nations established a regional network of national cancer institutes in 2011 to facilitate cooperation in advancing cancer control[9] (8).

Box 2.2. European Union policies for cancer[10] (9)
Prevention and screening
- Primary prevention: European Code against Cancer (see Box 3.4); regulation of carcinogens in the environment, food, at work, tobacco products
- Screening: Council recommendation for population-based cancer screening and support in implementation
Diagnosis and treatment
- Regulation of medical technologies
- Cross-border services (e.g. tele-radiology, provision of radioisotopes)
- Cross-border care (e.g. reference networks for rare diseases)
- Cross-border care financing (e.g. European health insurance card)
- Regulation of qualification of health care professionals
- Orpha.Net (information portal for rare cancers and diseases)
- Clinical guidelines (e.g. nutrition for cancer patients)
- Anti-discrimination protection for cancer patients and survivors under legislation on disability
Monitoring and research
- European cooperation for data on health services and outcomes (e.g. Eurostat) and cancer-specific studies, (e.g. EUROCARE)
- European Network of Cancer Registries • Financing of European research on cancer
- Regulation on use of personal data
- Regulation of clinical trials
Policy and infrastructure
- Overall policy statements by the Council of Ministers and European Parliament on cancer
- Financing of cooperation between Member States on cancer, including multiple joint actions
- European guidance on comprehensive cancer control strategies
- Financial support to health infrastructure (e.g. from European Structural and Investment Funds, European Investment Bank)
2.2.2 Guiding principles of a national response
Cancer cannot be prevented or controlled by the health sector alone.
The prevention and control of cancer require a coordinated, multisectoral, multidisciplinary approach.
Multisectoral collaboration and health-in-all policies are necessary for many of the most powerful NCD interventions, such as taxation of tobacco and unhealthy products, mitigation of air pollution and access to expensive medicines and technologies.
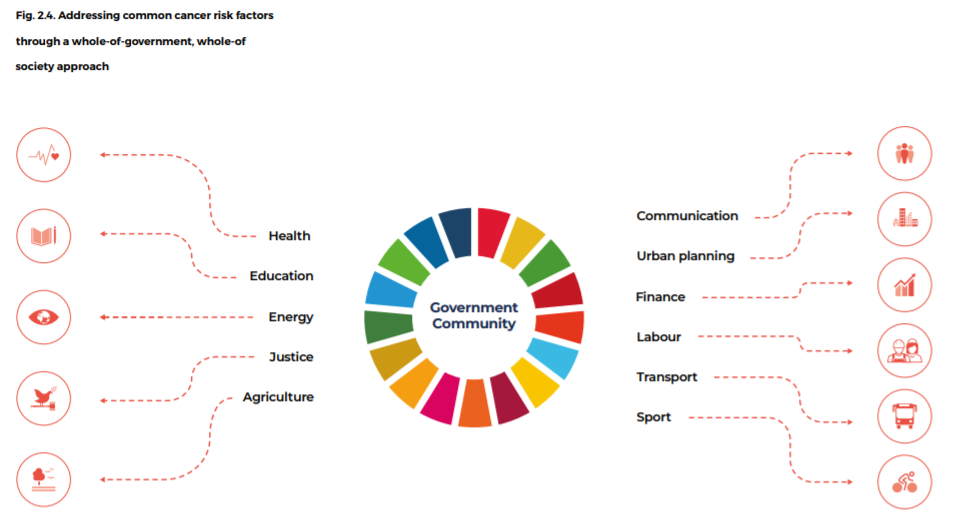
Cancer control is thus an excellent example of the whole-of-government, whole-of-society approach, with the leadership and involvement of many sectors, including health, education, energy, justice, agriculture, sports, transport, communication, urban planning, environment, labour, employment, industry, trade, finance and social and economic development (Box 2.3).

Box 2.3. Case study of tobacco control in Brazil
Brazil has a long-standing commitment to tobacco control. It is one of two countries with the highest achievement of all MPOWER policies.
Key measures implemented include designating all public and work places as 100% smoke-free, providing tobacco cessation services and strong pictorial health warnings, enforcing a comprehensive ban on tobacco advertising, promotion and sponsorship through Federal law and raising taxes on tobacco to 83% of the retail price.
The result is that the prevalence of smoking among adults decreased from 35% in 1989 to 10% in 2017.
Key drivers of change are strong political will, multisectoral action, with the involvement of civil society, investments in research, synthesis of evidence to inform policy, domestic resources and strong technical cooperation with WHO.
Brazil has also been involved in sub-regional forums to exchange experience and technical cooperation on tobacco taxation[11] (10).
A coalition of government ministries, public health institutes, insurance companies and health care providers are needed, with continuous support from stakeholders in civil society, voluntary and religious organizations and the private sector, as appropriate (Fig. 2.4).
Collaboration in promoting and supporting the objectives of cancer control should be strengthened (Box 2.4). In this whole-of-society perspective, alliances improve implementation of programmes and policies.
Fig. 2.4. Addressing common cancer risk factors through a whole-of-government, whole-of society approach

Box 2.4. Knowledge transfer between countries in Africa
In a South-South partnership, the Uganda Cancer Society provided technical assistance to stakeholders in Eswatini (previously Swaziland) in setting up and operating a cancer unit in Mbabane Government Hospital.
A formal arrangement was established, coordinated by the African Palliative Care Association and supported by the American Cancer Society.
The cancer unit now provides services for patients with breast cancer, with plans to extend services for other cancers.
The main interventions were exchange visits of experts, with on-the-job training and mentorship of staff at the Hospital and visits to Uganda for the lead pharmacist and a doctor for both observation and application of knowledge and skills[12] (11).
Towards achievement of UHC: UHC is the defining public health priority on the 2030 Agenda for Sustainable Development.
Its components are equitable access to services along the cancer continuum and financial protection for the whole population.
The response to the NCD burden should be based on primary health care and UHC and guided by human rights to promote equity, empowerment of peoples and communities, international cooperation and solidarity and multisectoral action in a life-course approach.
To succeed, cancer control must be integrated into the broader health system, and the investments made should also strengthen the health system (see also sections 3.4 and 7.2).
As recommended in the World Health Assembly resolution on cancer, an integrated approach should be used in which cancer control programmes are aligned with the broader NCD agenda Experience has shown that, when small initial investments in cancer care are oriented towards UHC, they ensure the feasibility and value of cancer services, improve population outcomes and justify the addition of services over time[13] (12) (Box 2.5).

Box 2.5. Case study of cancer in UHC agenda (Kazakhstan)
Kazakhstan has made a strong political commitment to cancer control and UHC founded on strong primary health care.
After an imPACT mission in 2016, WHO was requested to review the cancer programme and to identify interventions that would maintain or increase coverage of cancer services, provide value for money and ensure financial protection.
WHO and the Ministry of Health reviewed the country’s screening programmes and concluded that focusing on three evidence-based programmes would have more impact than six. In reviewing the country’s cancer treatment standards, WHO enlisted support from the European Society for Medical Oncology (ESMO), which analysed 20 cancer disease settings (over 300 protocols) using the WHO EML, the European Medicines Agency’s medicine indications, the ESMO Clinical Practice Guidelines[14] (13) the ESMO-Magnitude of Clinical Benefit Scale version 1.1 and expert peer review[15] (14).
The assessment supported the Ministry of Health in optimizing its cancer treatment protocols and linking them to the national EML.
Screening coverage increased from 60% to 90% for breast and cervical cancer, and treatment coverage increased from 85% to 89%.
By incorporating the recommendations into the Kazakhstan national cancer control plan 2018 -2022, the country maintained its long-standing commitment to offer its citizens evidence-based comprehensive cancer care as part of UHC[16] (15).
The response to the NCD burden should be based on primary health care and UHC and guided by human rights to promote equity, empowerment of peoples and communities, international cooperation and solidarity and multisectoral action in a life-course approach.
To succeed, cancer control must be integrated into the broader health system, and the investments made should also strengthen the health system (see also sections 3.4 and 7.2).
As recommended in the World Health Assembly resolution on cancer, an integrated approach should be used in which cancer control programmes are aligned with the broader NCD agenda through primary prevention, coherence is promoted in national cancer plans within broader health strategies, and horizontal integration ensures that cancer services are delivered as part of a comprehensive package at appropriate levels of care, with a focus on primary care.
Scaling up diagnostic imaging, laboratory capacity, infection prevention and control or palliative care can strengthen the whole health system for delivering other disease-specific programmes. Cancer control can be an indicator of a health system’s capacity and serve as an entry point for broader investment in the system.
2.3 Using a legal framework
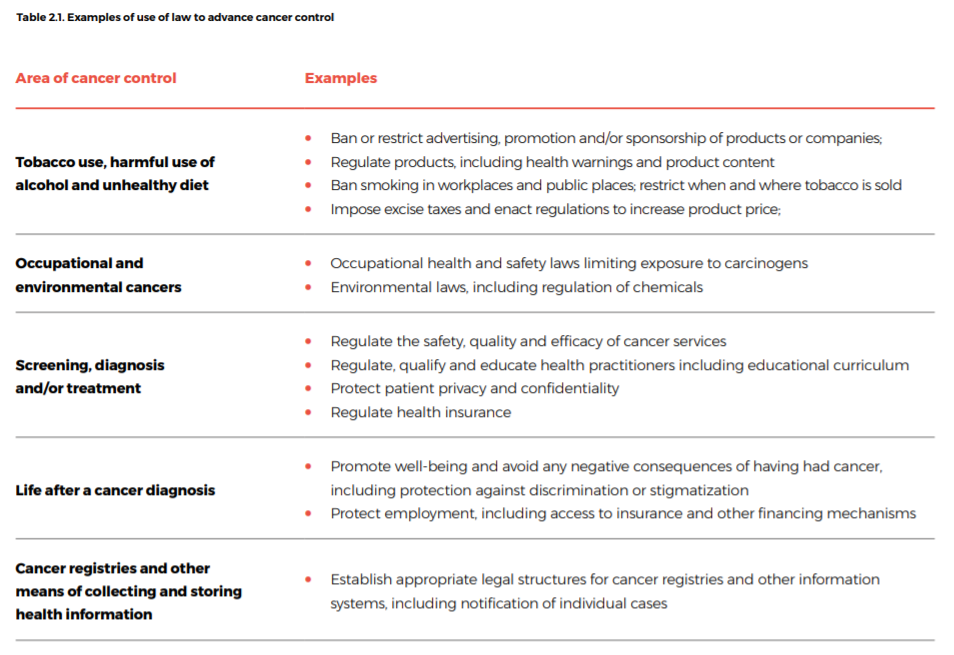
Many legal and regulatory frameworks have been used globally and locally to fulfil political commitments and for implementation, and national laws and regulations have been enacted to strengthen cancer control (Table 2.1).
Table 2.1. Examples of use of law to advance cancer control
Imbalances may occur in attempting to achieve coherence among different aspects of policy and practice. These include:
· liberalization and promotion of international trade and investment and regulation of unhealthy products, such as tobacco, alcohol and foods with excessive saturated fats, trans-fatty acids, salt or sugar;
· intellectual property protection, which can incentivize research and development, and the price of medicines and technologies;
· regulation of the trade and distribution of controlled medicines and the availability of opioids, which is essential for the relief of pain;
· laws designed to protect personal information and population-level cancer research; and
· material obtained for diagnostic purposes that could be used for research and the requirements of informed consent and linkage with clinical databases.
Legal and regulatory actions must be implemented and enforced.
For example, legislation to ensure that cancer is a reportable disease will increase the availability of data on incidence; however, resources should also be available for checking data for consistency and quality and for analysing them.
Legislative acts can authorize government agencies to formulate and implement programmes, as done in the Republic of Korea[17] (17).
While legislation to ensure access to treatment demonstrates government commitment, the capacity to make accurate diagnoses and provide survivorship care must be ensured (Box 2.6).

Box 2.6. Case study: Cancer law in the Philippines
The Philippines has effectively used health legislation to promote UHC. In March 2019, a bill on UHC was signed into law (Republic Act №11223), automatically enrolling all Filipinos in the National Health Insurance Program.
The bill built on previous legislation for UHC (the National Health Insurance Act of 1995), the Z Benefit Package of 2011 (for health conditions that require prolonged hospitalization and expensive treatment) and the Sin Tax Reform Law in 2012 on tobacco and alcohol.
Immediately before passing the UHC bill, the Government passed the National Integrated Cancer Control Act (Republic Act №11215) to strengthen cancer control, increase cancer survivorship and reduce physical and financial burdens on cancer patients and families.
Elements of the Act include creation of a cancer centre, a cancer assistance fund and a governance structure for multisectoral cancer control planning.
The goal of the legislation is attainment of the SDGs[18] [19] [20] (18–20).
International agreements that do not specifically address cancer but have a strong impact include the WHO Framework Convention on Tobacco Control (WHO FCTC) and the World Trade Organization Agreement on Trade-related Aspects of Intellectual Property Rights (TRIPS Agreement).
Under the WHO FCTC, a treaty negotiated under the auspices of WHO, 181 Parties are currently committed under international law to take evidence-based measures for tobacco control and to cooperate to achieve the aims of the convention. Since its entry into force in 2005, the WHO FCTC has supported and empowered implementation of tobacco control measures around the globe.
It was followed by the WHO FCTC Protocol to Eliminate Illicit Trade in Tobacco Products, the first protocol to the WHO FCTC, adopted in 2012, that was approved to reduce smuggling and other forms of illicit trade[21] (21).
The WHO FCTC is now integrated into the SDG agenda as one of the “means of implementation” to reach the overall health goal (SDG 3) and a target on NCDs.
Domestic implementation of the WHO FCTC has often been challenged in legal disputes, including claims that tobacco control measures violate other international commitments.
Prominent examples include legal challenges to tobacco packaging laws in Australia and Uruguay, where it was argued that the proposals violated the TRIPS Agreement.
The TRIPS Agreement, concluded in 1994, sets minimum standards for the protection of intellectual property, including patents and trademarks.
These requirements can intersect with public health goals, such as when governments restrict use of tobacco trademarks or seek to promote access to patented medicines. In November 2001, the World Trade Organization Ministerial Conference adopted the Doha Declaration on the TRIPS Agreement and Public Health, which states that “the TRIPS Agreement does not and should not prevent members from taking measures to protect public health … and, in particular, to promote access to medicines for all”.
Member states have also agreed to protect intellectual property under various bilateral and multilateral trade and investment agreements.
2.4 Why act now? Understanding the business case
Many countries are in the process of defining guaranteed health benefits packages for UHC, to explicitly define the rights and responsibilities of the population in accessing services.
This process often involves the use of economic data to quantify the value for money and budgetary impact of different intervention options, which reflect the necessity of the health sector to justify expenditures, particularly in relation to other sectors.
Additionally, investment cases are used to show the economic benefits of investing in particular services, either for specific diseases or for UHC as a whole.
Investment cases facilitate discussions between health and finance ministries regarding increasing the health budget for better, more responsible use of government resources.
WHO has drawn up an investment case for NCD prevention and control, Saving lives, spending less: a strategic response to noncommunicable diseases[22] (22), which shows that for every US$ 1 invested in scaling up interventions to address NCDs in LMIC, there will be a return to society of at least US$ 7 in increased employment, productivity and longer life.
US$ 1 invested in scaling up interventions to address NCDs in LMIC, there will be a return to society of at least US$ 7 in increased employment, productivity and longer life.
The interventions are now known as “best buys” and endorsed by member states[23] (23). Achievement of the SDG target of UHC would cost an estimated additional US$ 371 billion per year in LMICs[24] (24), equivalent to an additional investment of US$ 58 per person per year in all LMICs; however, this estimate included only a limited set of cancer management services.
Other groups have defined essential packages of services based on systematic reviews of published studies[25] [26](25, 26). Interventions such as management of childhood cancer are considered priorities in countries at all income levels.
As LMICs face an increasing need for cancer care services with continuation of the epidemiological transition, comprehensive planning for increased budgetary space for cancer services will become a necessity (see Box 7.1).
Thus, a new investment case solely for cancer care, with three progressive tiers of service, has been produced (Box 2.7).
2.4.1 Current investments in cancer
In 2016, the world spent US$ 7.5 trillion on health, representing almost 10% of global GDP[27] (24).
The average per capita expenditure was US$ 1000, but more than half of all countries spent less than US$ 350 per person.
To date, in many LMIC, domestic and external investments in cancer management have been insufficient, resulting in avoidable deaths[28] (27).
The unmet burden of cancer has both health and economic consequences at global, country, household and individual levels, resulting in hundreds of billions of dollars in economic loss each year (see section 1.2.2).
At country level, the lower the coverage of cancer care services, the greater the potential economic loss. Each individual who is unable to work or who dies prematurely as a result of cancer represents a loss of workforce participation, GDP contribution and human capital.

Box 2.7 Methodology for an investment case for cancer
Understanding the resources required, both financial and physical, to scale up cancer services is essential for negotiations between health and finance ministries on expanding the fiscal space for health, resulting in increasing financing for cancer services.
In this model, the additional resources required globally to scale up cancer services were estimated for eight cancers, of the breast, cervix, prostate, colon, rectum, lung, liver and stomach.
These cancers correspond to 54% of all cancer cases and 58% of all deaths; the estimate would likely increase with the addition of other cancers.
In order to focus on unmet needs, HIC, where cancer services are generally close to comprehensive and accessible, are excluded.
At three tiers of capacity (see section 6.5), resource-stratified packages of care were estimated as a basis for a phased approach for implementing cancer prevention and control plans.
Tiers are assigned according to a country’s health system.
In these calculations we modelled scale-up package 1 for LIC and package 2 for middle-income countries (MIC) to reach 90% of current unmet need by 2030.
This ambitious pattern is aligned with the ambitious scenario modelled in the Global cervical cancer elimination initiative investment case[29] (30).
In addition, we assumed that the scale up will have the added benefit of shifting the stage distribution at diagnosis, which is included in the health benefits calculation[30] (31).
Data on baseline coverage were taken from the same source as those for cervical cancer elimination[31] (30).
For countries for which data were not available, we assumed a current coverage of 20%, which reflects the current average coverage in LMIC.
All costs associated with delivering care are included, regardless of who currently pays for them. Costs are calculated in a bottom-up approach in which all the ingredients required to deliver an intervention are identified, the quantity of each is estimated and the price of each ingredient sought from global databases.
For human resource and facility costs, we used the WHO CHOICE global databases, inflated to 2020[32] [33](32,33). Medicine prices are taken from the Drug Price Indicator Guide and also inflated to 2020 values[34] (34).
The health impact associated with scaling up the package of interventions is calculated in a multistage life-table model. Epidemiological data for each cancer are taken from the IARC Global Cancer Observatory database[35] (35).
Effect sizes associated with the scaling-up of each intervention were derived from systematic literature searches and are described in chapter 6 on the resource-stratified packages.
To calculate the economic return on investment, two parameters aligned with previous investment case analyses are estimated[36] [37](36,37).
First, the productivity gains associated with reductions in mortality due to cancer are estimated. Each person whose life is saved is able to be an active member of the workforce and contribute to GDP at the average wage rate of the population[38] (38).
Secondly, each life saved results in broader societal benefits beyond direct workforce participation. This value is quantified by a metric known as the value of statistical life, which is the amount an individual would be willing to pay to avoid death. For this analysis, we assume that the statistical value of a life is 22 x GDP per capita per life saved[39] (39). While the long-term aim is comprehensive packages of care available for all countries as modelled, this may be unachievable in the short term for some countries.
We therefore also provide a less ambitious scenario, in which the same packages of services are modelled to reach 50% of unmet need by 2030, with no improvement in stage distribution from the country baseline.
The trade-off is the number of lives saved, which will be only 400 000 per year by 2030 or a total of 2.2 million over 10 years.
This increase is unlikely to allow these countries to achieve the SDG of reducing premature mortality due to NCDs by one third.
2.4.2 Additional investment needs for cancer control programmes
The cancer investment case indicates that an ambitious scale up to 90% coverage of cancer services by 2030 would cost US$ 140 billion between 2020 and 2030.
As coverage increases, so does cost, and the additional investment required will be US$ 2.5 billion in 2021 and up to an additional US$ 25.3 billion in 2030.
This invesment corresponds to US$ 4.05 per person per year by 2030; this includes the US$1.65 per capita captured in the SDG costing for colorectal, cervical and breast cancers and an additional US$2.40 for the other four cancers, representing a 4% increase to the previous estimate of US$ 58 per person required to attain the health-related SDGs[40] (37).
The greatest investment is needed for additional human resources to deliver health services for priority packages in tiers 1 and 2 (Fig. 2.5).
Medicines constitute the second greatest share. Package 3, which includes many of the high-cost cancer medications, is not included in the priority packages selected for LMIC.
The additional human resources required may represent a hindrance to rapid scaling-up of these interventions, given the long pre-service training necessary for the many highly specialized services.
Fig. 2.5. Percentage contribution of different costs to priority cancer packages in LMIC.
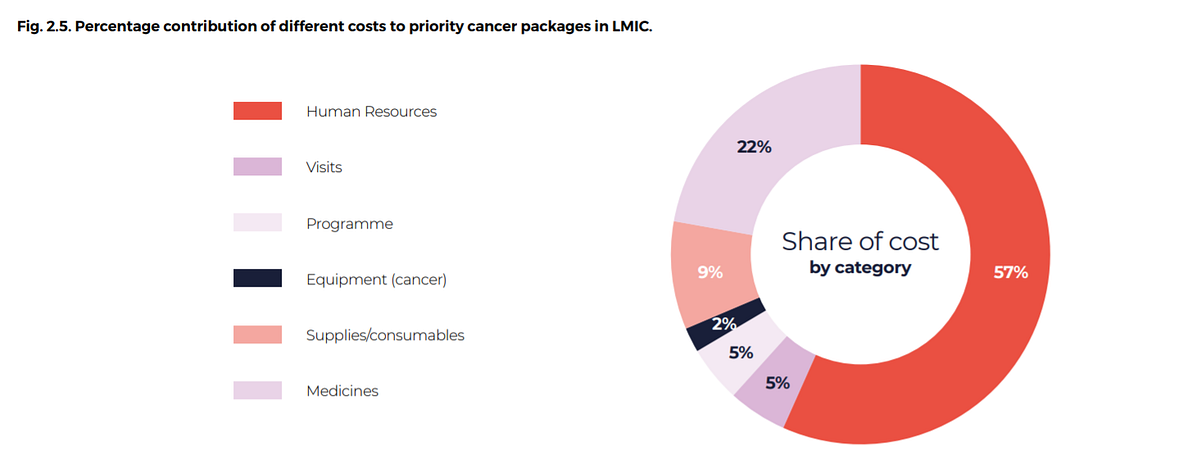
Fig. 2.6. Per capita investments needed for cancer management scale-up by World Bank country income level (expressed in US$ and based on 2018 population estimates)
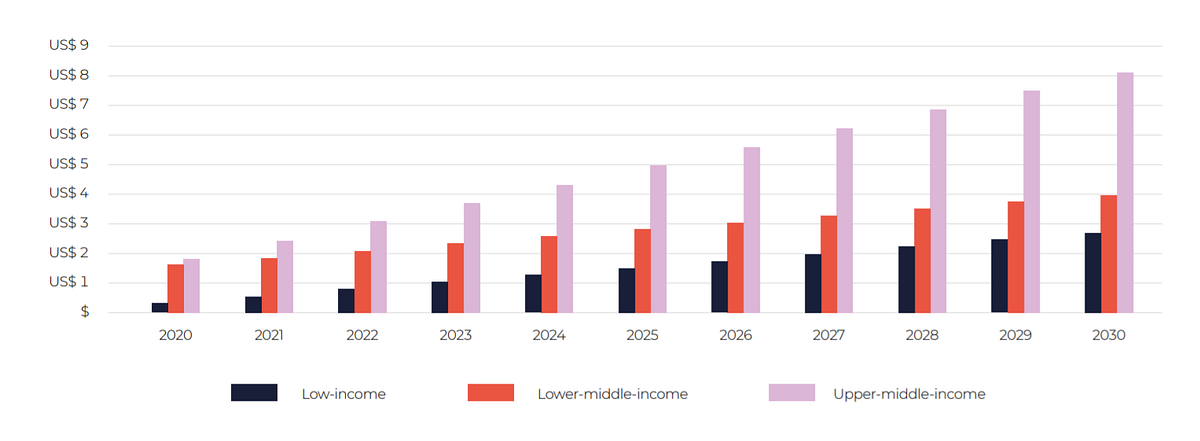
The per capita investment required varies by country, partly because of the expensive package modelled for MIC and partly because of the higher costs of non-traded goods in those countries.
By 2030, the investment required will be US$ 2.70 per person in LIC, US$ 3.95 per person in lower-middle-income countries and US$ 8.15 per person in upper -middle-income countries (Fig. 2.6).
2.4.3 Health impacts of investing in cancer control programmes
This ambitious target for rapid scale up would save 1.1 million lives per year in LMIC in 2030 (Fig. 2.7).
Over the course of the 10-year investment, 7.3 million lives can be saved.
The numbers of lives saved depend on the cancer type, the baseline burden of disease and the effectiveness of interventions.
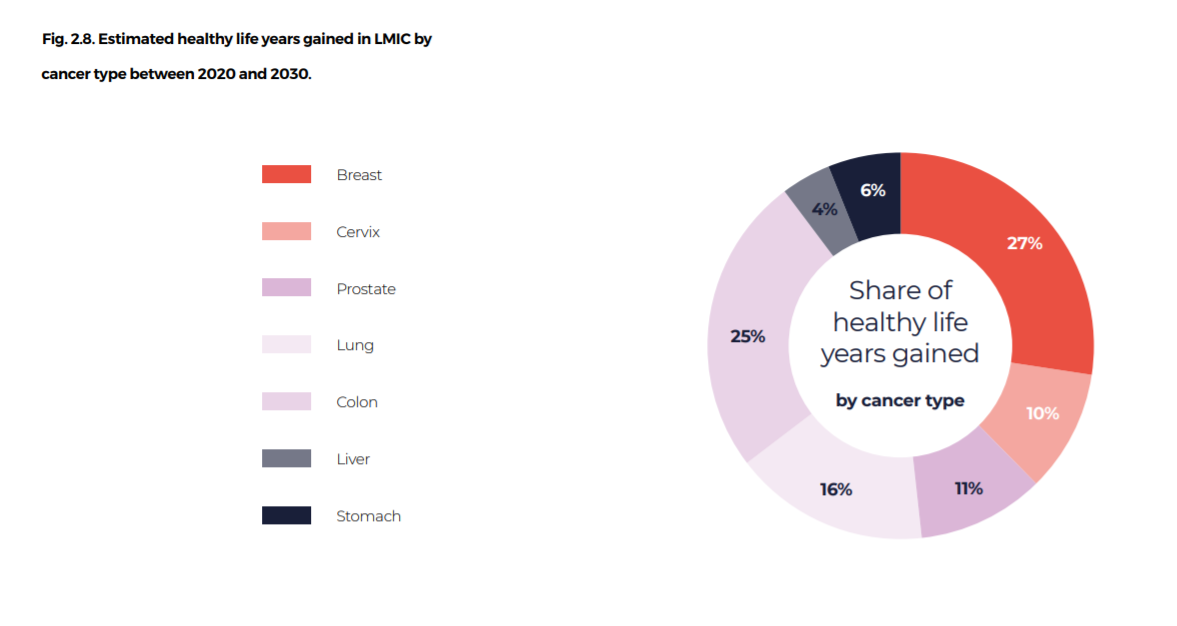
Populations in LMIC will not only live but will also become healthier, with 10 million years of healthy life added to the population between 2020 and 2030 (Fig. 2.8).
Fig. 2.7. Projected numbers of lives saved for each common cancer type over the next 10 years
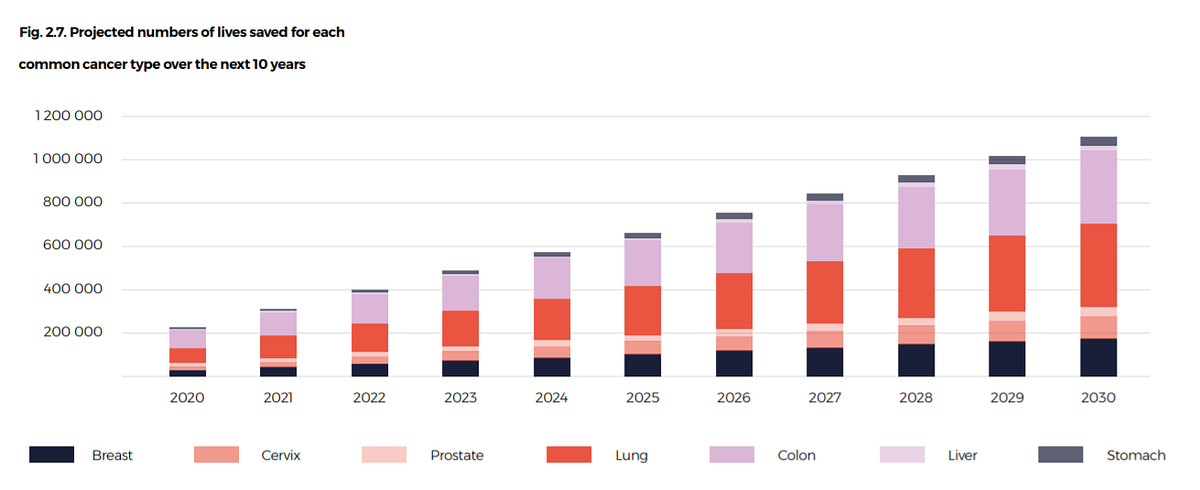
Fig. 2.8. Estimated healthy life years gained in LMIC by cancer type between 2020 and 2030.
2.4.4 How will economies benefit?
While health benefits alone are a convincing basis, economic rationales are increasingly used to strengthen the case for additional investment in health care.
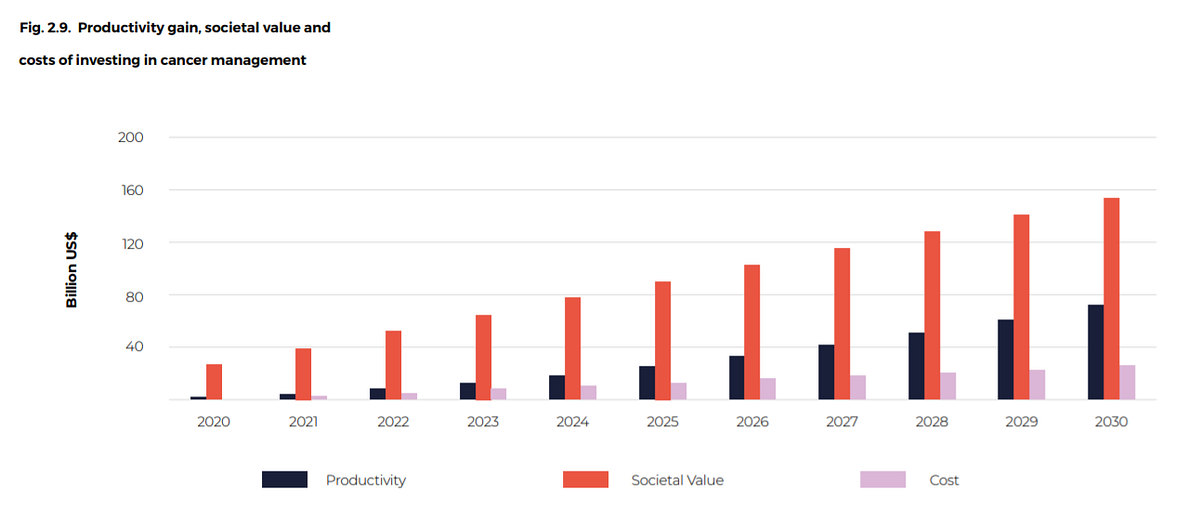
Investments will not only keep people alive but will increase both direct workforce participation and a broader societal contribution, adding US$ 325 billion in direct productivity gains over the next 10 years and US$ 990 billion in indirect societal gains, for a full social value of US$ 1.315 trillion.
This is equivalent to a direct productivity return of US$ 2.30 for each US$ 1 invested in cancer care and a full social return based on both direct productivity and societal gains of US$ 9.50 (Fig. 2.9). Box 2.8 illustrates the consequences of investing less.
Fig. 2.9. Productivity gain, societal value and costs of investing in cancer management
Investing in cancer control makes sense from both a health and an economic viewpoint.
Scaling-up access to health services and ensuring that access is free from financial barriers will improve quality of life and reduce mortality rates.
This will lead to a more productive society, with greater workforce participation and a strengthened social fabric.
Fig 2.8. Return on investment


Box 2.8. What will happen if less is invested?
While the long-term aim is for all countries to have comprehensive packages of care available, some countries may not be able to achieve this in the short term.
A less ambitious scenario was therefore prepared, scaling up the same packages of services to reach 50% of unmet need by 2030. In this scenario, the additional investment required is much lower and, in some settings, possibly more affordable, at only US$ 1.70 per person per year by 2030.
The trade-off is the number of lives saved, which will be only 400 000 per year by 2030 or a total of 2.2 million over 10 years. This increase is unlikely to allow these countries to achieve the SDG of reducing premature mortality due to NCDs by one third.
These estimates are intended to highlight the possible health benefits in LMIC resulting from investment in cancer control programmes.
The 90% coverage scenario gives countries an ambitious option and the 50% coverage scenario is an alternative, lower investment option.
Ultimately, countries should rely on their own data and priorities for deciding where to spend their limited health budgets (see section 7.4.4, Box 7.1).
References
See the original publication.
Suggested citation
WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
Originally published at https://www.who.int