Health, Care and Tech Transformation (HCTT)
research institute, knowledge portal and
advisory consulting
Joaquim Cardoso MSc*
Chief Researcher & Editor and Chief Advisory Consulting
November 5, 2022
MSc* from London Business School
MIT Sloan Masters Program
Find below an analysis by Daniel Kaul, MD, at NEJM Journal Watch, reviewing Ben Abdallah S et al. Clin Infect Dis 2022 Nov 4, Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial, Clinical Infectious Diseases, 2022;, ciac807, https://doi.org/10.1093/cid/ciac807
On the second part of the post, you find an abstract of the reference publication.
ORIGINAL PUBLICATION

Zinc for Patients with COVID-19?
NEJM Journal Watch
Daniel Kaul, MD, reviewing Ben Abdallah S et al. Clin Infect Dis 2022 Nov 4
December 2, 2022
In a randomized, blinded, multicenter study in Tunisia, oral zinc demonstrated a beneficial effect on COVID-19 progression, particularly in hospitalized patients with severe disease.
Since the beginning of the COVID-19 pandemic, initial reports of improved outcomes with various repurposed drugs or vitamins (e.g., vitamin C, hydroxychloroquine) have not been confirmed in larger randomized studies.
Since the beginning of the COVID-19 pandemic, initial reports of improved outcomes with various repurposed drugs or vitamins (e.g., vitamin C, hydroxychloroquine) have not been confirmed in larger randomized studies.
Now, investigators report results of a randomized trial of zinc.
- Between February 15 and May 4, 2022,
- 470 patients presenting to the COVID-19 triage area at
- five hospitals in Tunisia were randomized to 15 days of oral zinc (25 mg twice daily) or placebo.
- Patients with severe comorbidities, need for assisted ventilation, or >7 days of symptoms were excluded.
- Mean age was 54, 60% required supplemental oxygen at enrollment, and 23% had received at least one dose of COVID-19 vaccine.
At 30 days, the primary composite outcome (ICU admission or death) occurred in 10.4% and 16.7% of the zinc and placebo groups, respectively.
Length of hospital stay was 3.5 days shorter in zinc recipients.
Ambulatory patients who received zinc reported shorter duration of symptoms, but no reduction in need for hospital admission.
At 30 days, the primary composite outcome (ICU admission or death) occurred in 10.4% and 16.7% of the zinc and placebo groups, respectively.
Length of hospital stay was 3.5 days shorter in zinc recipients.
Ambulatory patients who received zinc reported shorter duration of symptoms, but no reduction in need for hospital admission.
COMMENT
Most COVID-19 trials enroll either hospitalized patients with severe disease or outpatients with mild to moderate disease.
This study included both groups, making interpretation more challenging. Performing a confirmatory study would be difficult as high rates of death and ICU admission are not now occurring.
I’m not ready to recommend zinc for hospitalized patients with COVID-19 (or use it in lieu of proven outpatient treatments), but for patients or clinicians who wish to try zinc, there’s probably little downside — and now some scientific justification.
I’m not ready to recommend zinc for hospitalized patients with COVID-19 (or use it in lieu of proven outpatient treatments), …
… but for patients or clinicians who wish to try zinc, there’s probably little downside — and now some scientific justification.
CITATIONS
Ben Abdallah S et al. Twice-daily oral zinc in the treatment of patients with coronavirus disease 2019: A randomized double-blind controlled trial. Clin Infect Dis 2022 Nov 4; ciac807; [e-pub]. (https://doi.org/10.1093/cid/ciac807)
REFERENCE PUBLICATION (excerpt)

Oxford Academic
Clinical Infectious Diseases
Saoussen Ben Abdallah, Yosra Mhalla, Imen Trabelsi, Adel Sekma, Rim Youssef, Khaoula Bel Haj Ali, Houda Ben Soltane, Hajer Yacoubi, Mohamed Amine Msolli, Nejla Stambouli, Kaouthar Beltaief, Mohamed Habib Grissa, Meriem Khrouf, Zied Mezgar, Chawki Loussaief, Wahid Bouida, Rabie Razgallah, Karima Hezbri, Asma Belguith, Naouel Belkacem, Zohra Dridi, Hamdi Boubaker, Riadh Boukef, Semir Nouira
4 November 2022
ABSTRACT
Background
- Zinc supplementation has been considered a potential therapy for coronavirus disease 2019 (COVID-19).
- We aimed to examine zinc efficacy in adult patients with COVID-19 infection.
Methods
- We conducted a prospective, randomized, double-blind, placebo-controlled multicenter trial.
- Patients who were tested positive for COVID-19 without end-organ failure were randomized to oral zinc (n = 231) or matching placebo (n = 239) for 15 days.
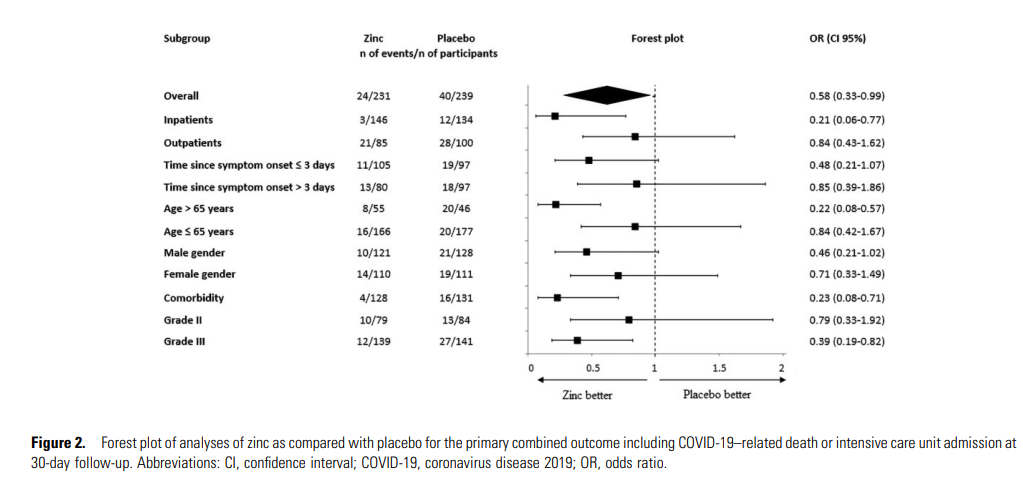
- The primary combined outcome was death due to COVID-19 or intensive care unit (ICU) admission ≤30 days after randomization.
- Secondary outcomes included length of hospital stay for inpatients and duration of COVID-19 symptoms with COVID-19–related hospitalization for outpatients.
Results
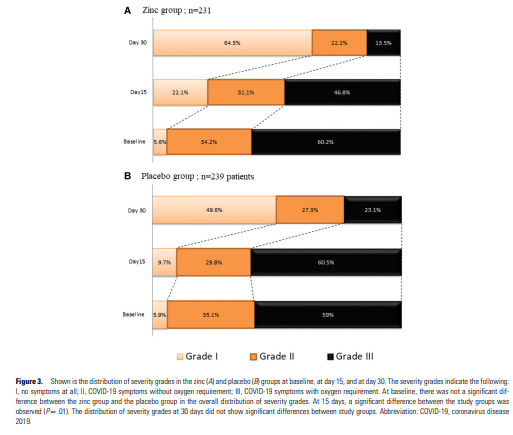
- 190 patients (40.4%) were ambulatory and 280 patients (59.6%) were hospitalized.
- Mortality at 30 days was 6.5% in the zinc group and 9.2% in the placebo group (OR: .68; 95% CI .34–1.35);
- ICU admission rates were, respectively, 5.2% and 11.3% (OR: .43; 95% CI .21–.87).
- Combined outcome was lower in the zinc group versus the placebo group (OR: .58; 95% CI .33–.99).
- Consistent results were observed in prespecified subgroups of patients aged <65 years, those with comorbidity, and those who needed oxygen therapy at baseline.
- Length of hospital stay was shorter in the zinc group versus the placebo group (difference: 3.5 days; 95% CI 2.76–4.23) in the inpatient group;
- duration of COVID-19 symptoms decreased with zinc treatment versus placebo in outpatients (difference: 1.9 days; 95% CI .62–2.6).
- No severe adverse events were observed during the study.
Conclusions
- Our results showed that, in COVID-19 patients, oral zinc can decrease 30-day death,
- ICU admission rate and
- can shorten symptom duration.
Infographic
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has created a global health crisis since its first emergence in China in late December 2019.
Its high transmissibility and increased morbidity have made COVID-19 a serious public health threat and burden [1].
Various drugs were initially proclaimed effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19; they were later disapproved [2].
Various drugs were initially proclaimed effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19; they were later disapproved [2].
Like in many other diseases, the regulation of white blood cell production using immuno-nutrition is a novel concept that could be applied to COVID-19.
Some molecules and nutrients such as zinc play central roles in keeping the function and integrity of the immune system [3, 4].
It has been shown that serum zinc was inversely correlated with outcome in sepsis, highlighting the potential value of the zinc approach in COVID-19 treatment [5–8].
Some molecules and nutrients such as zinc play central roles in keeping the function and integrity of the immune system [3, 4].
It has been shown that serum zinc was inversely correlated with outcome in sepsis, highlighting the potential value of the zinc approach in COVID-19 treatment [5–8].
Nevertheless, there is still limited evidence to support such a role of zinc in COVID-19, with the exception of observational studies and a few randomized clinical trials with small sample sizes [9–11].
The objective of the present trial was to evaluate the effect of zinc supplementation in non–critically ill patients with COVID-19.
Our a priori hypothesis was that supplementation with zinc would reduce 30-day mortality and need for intensive care unit (ICU) admission.
METHODS & ADDITIONAL INFORMATION
See the original publication
DISCUSSION
In this multicenter, randomized, double-blind trial involving patients with COVID-19, a significant and clinically meaningful decrease in 30-day ICU admission rate and shorter duration of COVID-19 symptoms were observed with oral zinc administered for 15 days.
In our prespecified subgroup analyses, we found that this benefit was especially observed in aged patients and those with comorbid conditions or those who need oxygen.
The activity of zinc against infectious pathogens has been demonstrated in a variety of viral species [12–16].
However, there is very scant information available on the role and effect of zinc in coronavirus disease [17–21].
Jothimani et al [18] in a recently published study including 47 patients with COVID-19 demonstrated that zinc deficiency was present in more than half (57.4%) of their cohort and was associated with prolonged hospitalization and increased mortality compared with a control group.
Jothimani et al [18] in a recently published study including 47 patients with COVID-19 demonstrated that zinc deficiency was present in more than half (57.4%) of their cohort and was associated with prolonged hospitalization and increased mortality compared with a control group.
In 4 outpatients with COVID-19, Finzi [19] reported that treatment with zinc reduced disease symptoms within 24 hours.
Several clinical trials are currently investigating the effects of various doses of zinc either alone or with ionophores on the initiation and the progression of COVID-19 in patients.
To our knowledge, this study is the first well-powered, placebo-controlled clinical trial to report results of zinc for the treatment of patients with COVID-19.
When administered orally to patients hospitalized with COVID-19 without end-organ failure, zinc demonstrated its efficacy to prevent ICU admission and to reduce hospital length of stay; …
… for outpatients, zinc reduced symptom duration.
Zinc should be considered for the treatment of patients with COVID-19.
When administered orally to patients hospitalized with COVID-19 without end-organ failure, zinc demonstrated its efficacy to prevent ICU admission and to reduce hospital length of stay; …
… for outpatients, zinc reduced symptom duration.
Zinc should be considered for the treatment of patients with COVID-19.
Major strengths of our trial include being a randomized controlled trial and the large sample size.
Moreover, subgroup analyses showed consistent results across both low- and high-risk patients.
For inpatients, although the risk of death among patients receiving zinc and placebo was similar,
- the absolute risk reduction in ICU admission was approximately 10% and
- the length of hospital stay was reduced by 3 days.
These results suggest that, for survivors, the disease trajectory is milder in zinc-group patients, with reduced need for hospital care and organ support.
In outpatients, zinc treatment was associated with
- a reduction in COVID-19 symptom duration by approximately 3 days;
- in addition, there was a trend for fewer hospital admissions in patients in the zinc group, but the difference was not significant because of the small sample size of this category of patients.
Our results should be highlighted with regard to not only the magnitude of a zinc positive effect but also the fact that this product is readily available at a low price to a larger percentage of the population, with minimal risk.
This trial has several limitations.
First, generalizability is limited beyond patients with moderate clinical severity.
Second, whether using zinc at higher doses than those prescribed in this trial would lead to different results is a question that needs to be investigated.
Of note, the dose of zinc used in our study is within the range that is currently recommended [22].
Of relevance, 50 mg of zinc per day was found to be tolerable and unlikely to induce toxicity.
Third, one could argue that longer treatment with zinc (>15 days) could add more clinical benefit. It is an important question, and we probably need specific data on long-term outcome and the possible preventive effect of zinc against the risk of long COVID [23].
The success of zinc treatment may be dependent on zinc serum levels; we did not assess serum zinc in the present study, and so we could not verify this relationship.
Finally, although follow-up via telephone contact does not replace face-to-face visits, it was possible for us to assess outcomes in almost all of our included patients.
The success of zinc treatment may be dependent on zinc serum levels; we did not assess serum zinc in the present study, and so we could not verify this relationship.
In summary,
- oral zinc treatment for 15 days is associated with a nearly 40% reduction in death and ICU admission,
- with shortening of symptom duration, in patients with COVID-19.
- Our results have very important clinical relevance in the absence of specific effective curative treatment.
Our results have very important clinical relevance in the absence of specific effective curative treatment.
References and additional information
See the original publication
About the authors & affiliations
Saoussen Ben Abdallah,1 Yosra Mhalla,2 Imen Trabelsi,3 Adel Sekma,3,4 Rim Youssef,3,5 Khaoula Bel Haj Ali,3,4 Houda Ben Soltane,3,6 Hajer Yacoubi,3,5 Mohamed Amine Msolli,3,4 Nejla Stambouli,7 Kaouthar Beltaief,3,4 Mohamed Habib Grissa,3,4 Meriem Khrouf,3,6 Zied Mezgar,3,6 Chawki Loussaief,8 Wahid Bouida,3,4 Rabie Razgallah,9 Karima Hezbri,10 Asma Belguith,11 Naouel Belkacem,12 Zohra Dridi,13 Hamdi Boubaker,3,4 Riadh Boukef,3,5 and Semir Nouira3,4
1 Medical Intensive Care Unit, Fattouma Bourguiba University Hospital, Monastir, Tunisia;
2 Laboratory of Microbiology, Fattouma Bourguiba University Hospital, Monastir, Tunisia;
3 Research Laboratory LR12SP18, University of Monastir, Monastir, Tunisia;
4 Emergency Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia;
5 Emergency Department, Sahloul University Hospital, Sousse, Tunisia;
6 Emergency Department, Farhat Hached University Hospital, Sousse, Tunisia;
7 UR17DN03 — Research Unit, Military Defense, Military Hospital of Tunis, Tunis, Tunisia;
8 Department of Infectious Disease, Fattouma Bourguiba University Hospital, Monastir, Tunisia;
9 DACIMA Consulting, Tunis, Tunisia;
10Opalia Recordati, Tunis, Tunisia;
11Department of Preventive Medicine, Fattouma Bourguiba University Hospital, Monastir, Tunisia;
12Emergency Department, District Hospital Teboulba, Teboulba, Tunisia; and
13Department of Cardiology, Fattouma Bourguiba University Hospital, Monastir, Tunisia
Cite
Saoussen Ben Abdallah, Yosra Mhalla, Imen Trabelsi, Adel Sekma, Rim Youssef, Khaoula Bel Haj Ali, Houda Ben Soltane, Hajer Yacoubi, Mohamed Amine Msolli, Nejla Stambouli, Kaouthar Beltaief, Mohamed Habib Grissa, Meriem Khrouf, Zied Mezgar, Chawki Loussaief, Wahid Bouida, Rabie Razgallah, Karima Hezbri, Asma Belguith, Naouel Belkacem, Zohra Dridi, Hamdi Boubaker, Riadh Boukef, Semir Nouira, Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial, Clinical Infectious Diseases, 2022;, ciac807, https://doi.org/10.1093/cid/ciac807