Chief Researcher and Editor:
Joaquim Cardoso MSc
the health transformation / journal
November 2, 2022
This is a republication of the paper below, with the title above.
Impact of Clinical Practice Gaps on the Implementation of Personalized Medicine in Advanced Non-Small-Cell Lung Cancer
JCO Precision Oncology
Helen Sadik, PhD1; Daryl Pritchard , PhD2; Derry-Mae Keeling, BSc1; Frank Policht, PhD1; Peter Riccelli, PhD1; Gretta Stone, BS3; Kira Finkel, MSPH3; Jeff Schreier, MBA1; and Susanne Munksted, MS1
October 31, 2022
ABSTRACT
Purpose
- Personalized medicine presents new opportunities for patients with cancer.
- However, many patients do not receive the most effective personalized treatments because of challenges associated with integrating predictive biomarker testing into clinical care.
- Patients are lost at various steps along the precision oncology pathway because of operational inefficiencies, limited understanding of biomarker strategies, inappropriate testing result usage, and access barriers.
- We examine the impact of various clinical practice gaps associated with diagnostic testing-informed personalized medicine strategies on the treatment of advanced non–small-cell lung cancer (aNSCLC).
Patients are lost at various steps along the precision oncology pathway because of operational inefficiencies, limited understanding of biomarker strategies, inappropriate testing result usage, and access barriers.
Methods:
- Using Diaceutics’ Data Repository, a multisource database including commercial and Medicare claims and laboratory data from over 500,000 patients with non–small-cell lung cancer in the United States, we analyzed the number of patients with newly diagnosed aNSCLC who could have, but did not, benefit from a personalized treatment.
- The analysis focuses on the independent and cumulative impacts of gaps occurring during seven steps of the precision oncology pathway, from diagnosis to treatment.
Results
- For every 1,000 patients in the study cohort, 497 (49.7%) are lost to precision oncology because of factors associated with getting biomarker test results.
- Among the 503 of 1,000 patients who did receive results from a biomarker test, 147 (29.2%) did not receive appropriate targeted treatments.
- Thus, approximately 64% of potentially eligible patients with aNSCLC are not benefiting from precision oncology therapies appropriate for their disease.
… approximately 64% of potentially eligible patients with aNSCLC are not benefiting from precision oncology therapies appropriate for their disease.
Conclusion
- Most patients with aNSCLC eligible for precision oncology treatments do not benefit from them because of clinical practice gaps.
- This finding is likely reflective of similar gaps in other cancer types.
- An increased understanding of the impact of each practice gap can inform strategies to improve the delivery of precision oncology, helping to fully realize the promise of personalized medicine.
An increased understanding of the impact of each practice gap can inform strategies to improve the delivery of precision oncology, helping to fully realize the promise of personalized medicine.
Practice Gaps [excerpted from the paper]
The analysis focused on the independent and cumulative impact of the gaps occurring during seven discrete steps of the precision oncology pathway from diagnosis to treatment.
These practice gaps encompass barriers observed at each step, broadly summarized as follows:
- Step 1: Biopsy referral: Initial solid or blood biopsy was never performed.
- Step 2: Biospecimen collection: Biospecimen collection challenges including insufficient tissue or tumor cell content of initial biopsy or rebiopsy inhibited biomarker testing and its accuracy.
- Step 3: Biospecimen evaluation/pathology: Biospecimen tumor cell content was overestimated, inhibiting biomarker testing and its accuracy.
- Step 4: Biomarker test ordering: Appropriate testing was not ordered, or treatment began before testing was ordered.
- Step 5: Biomarker testing performance: Biomarker testing provided inconclusive or false-negative (FN) results.
- Step 6: Test result reporting: As a result of turnaround time (TAT) delays, treatment was initiated without consideration of test results.
- Step 7: Treatment decision: Targeted treatment was not selected despite positive test results.
CONTEXT
Key Objective
- This article reflects a large multisource US commercial and Medicare claims and laboratory database analysis of the number of patients with newly diagnosed advanced non-small-cell lung cancer (NSCLC) who could have, but did not, benefit from a personalized treatment because of various clinical practice gaps occurring during the delivery of precision oncology care, from diagnosis to treatment.
Knowledge Generated
- We examine clinical practice gaps along the precision NSCLC treatment pathway and quantify the loss of patients because of each clinical gap, including preanalytic biomarker testing and post-analytic practice challenges, ultimately showing that approximately 64% of potentially eligible patients with advanced NSCLC are not benefiting from precision oncology therapies appropriate for their disease.
Relevance
- Improving the implementation of precision oncology care requires a better understanding of the associated clinical practice gaps and their impact on patient care.
ORIGINAL PUBLICATION

Impact of Clinical Practice Gaps on the Implementation of Personalized Medicine in Advanced Non–Small-Cell Lung Cancer
JCO Precision Oncology
Helen Sadik, PhD1; Daryl Pritchard , PhD2; Derry-Mae Keeling, BSc1; Frank Policht, PhD1; Peter Riccelli, PhD1; Gretta Stone, BS3; Kira Finkel, MSPH3; Jeff Schreier, MBA1; and Susanne Munksted, MS1
October 31, 2022
Introduction
Precision oncology strategies are an important and growing component of cancer care.
There are over 90 US Food and Drug Administration-approved targeted therapies available for use in eligible patients with cancer,1and a recent oncology pipeline report showed that approximately 55% of all oncology clinical trials involved the use of biomarkers.2Predictive biomarker testing to help identify patients who could benefit from targeted therapies is a cornerstone of personalized medicine in cancer care, allowing for more rapid diagnosis while informing treatment decisions that could lead to better patient outcomes and systemic efficiencies.
The success of precision oncology relies on the accurate identification of patients harboring biomarker alterations as determined by laboratory test results subsequently used to guide therapeutic decisions.
Biomarker testing and targeted therapeutics are relatively new, however, and providers face several challenges in adapting cancer care practices accordingly. Levels of biomarker testing differ greatly across practice settings, tumor types, and biomarkers, with varying adherence to testing guidelines.3Implementation challenges are exemplified in non-small-cell lung cancer (NSCLC), where although more than 70% of patients have tumors with biomarker alterations related to therapeutic options,4many patients still do not receive biomarker testing.3,5
Furthermore, many cancer patients with actionable results as determined by biomarker testing do not actually receive appropriate targeted therapies.
Reports show that more than one third of US patients with cancer miss out on precision oncology treatment because of suboptimal testing practices specifically related to quality or sample management issues.6,7For NSCLC, studies examining practice-based data from over 190 US hospital systems have estimated that only 65%-75% of patients with an actionable mutation actually receive targeted therapies.8–10Another study examining patients with NSCLC within the Veterans Affairs National Precision Oncology Program revealed that more than 30% of patients with highly actionable gene variants received chemotherapy instead of more effective targeted treatments.11
Testing and treatment misfires in precision oncology have clinical consequences.
An examination of a large registry claims database from 2010 through 2018, for example, showed that a significant percentage of patients with advanced epidermal growth factor receptor-positive ( EGFR+) and anaplastic lymphoma kinase-positive ( ALK+) NSCLC who did not receive targeted therapies had inferior survival rates.12
An examination of a large registry claims database from 2010 through 2018, for example, showed that a significant percentage of patients with advanced epidermal growth factor receptor-positive ( EGFR+) and anaplastic lymphoma kinase-positive ( ALK+) NSCLC who did not receive targeted therapies had inferior survival rates.12
These inefficiencies may exemplify the clinical challenges of navigating the complex precision oncology pathway, which includes multiple steps from diagnosis to treatment.
At various steps, clinical practice gaps caused by operational inefficiencies, limited awareness or understanding of biomarker strategies, inappropriate use of testing results, and coverage and payment challenges can lead to missed opportunities for patients to benefit from targeted treatments. These clinical practice gaps can occur during preanalytic stages of diagnostic testing related to biopsy collection and evaluation and test ordering. They can also occur during analytical and postanalytic stages related to test performance, result reporting, and treatment decisions.
Implementing consistent biomarker testing-informed precision oncology care requires a better understanding of the associated clinical practice gaps and the impact each gap has on patient care.
In this study, we used laboratory and claims-based data from the US health system to examine clinical practice gaps associated with diagnostic testing-informed personalized medicine strategies in NSCLC and quantified the loss of patients along the precision oncology pathway because of each practice gap.

Methods
To quantify the extent to which clinical practice gaps are affecting the treatment of patients with advanced NSCLC (aNSCLC) in the US health care setting, …
… data from a large population of newly diagnosed patients were analyzed to estimate the number of patients who could have, but did not, benefit from a personalized treatment.
The analysis focused on the independent and cumulative impact of the gaps occurring during seven discrete steps of the precision oncology pathway from diagnosis to treatment.
These practice gaps encompass barriers observed at each step, broadly summarized as follows:
- Step 1: Biopsy referral: Initial solid or blood biopsy was never performed.
- Step 2: Biospecimen collection: Biospecimen collection challenges including insufficient tissue or tumor cell content of initial biopsy or rebiopsy inhibited biomarker testing and its accuracy.
- Step 3: Biospecimen evaluation/pathology: Biospecimen tumor cell content was overestimated, inhibiting biomarker testing and its accuracy.
- Step 4: Biomarker test ordering: Appropriate testing was not ordered, or treatment began before testing was ordered.
- Step 5: Biomarker testing performance: Biomarker testing provided inconclusive or false-negative (FN) results.
- Step 6: Test result reporting: As a result of turnaround time (TAT) delays, treatment was initiated without consideration of test results.
- Step 7: Treatment decision: Targeted treatment was not selected despite positive test results.

Data Source and Population
Analysis was based on Diaceutics’ proprietary DXRX Data Repository, a multisource database that consists of commercial and Medicare claims and laboratory data.
The data set contains real-time laboratory data, deidentified at a patient level, and covers 340 million lives. The data repository was developed in 2015 and is continuously updated weekly and is funded by Diaceutics.13Data contain patient diagnosis code; physician national provider identifier; laboratory-performed, test and panel name; result of test (positive, negative, quantity not sufficient, test not performed, inconclusive, etc); and any specific genomic alterations (eg, mutations, fusions, etc) detected. The repository covers 84% of patients with lung cancer whose data are included in the US National Cancer Institute’s SEER Program database.14
Within the Medicare claims portion of the repository, a population of 38,068 patients with aNSCLC newly diagnosed and actively managed in 2019 were identified for practice gap analyses.
Demographics of the patients included in this study are summarized in Table 1. Actively managed patients were defined as having three or more appearances/procedures noted in claims data, thereby providing enough data to construct a clear picture of their clinical journeys. Patients were further categorized on the basis of whether they had a biopsy performed (tissue or liquid); their biomarker testing methodology (immunohistochemistry [IHC], fluorescence in situ hybridization, next-generation sequencing [NGS], Sanger sequencing, or polymerase chain reaction [PCR]); and their treatment category (chemotherapy, immunotherapy, or targeted). Patients who were diagnosed or underwent testing in the final 3 months of 2019 often had incomplete treatment data and were removed from treatment analysis. The type of therapy received was evaluated using claims associated with Medicare Parts A/B (inpatient/outpatient/non-hospital-based care provision) and D (prescription drugs).
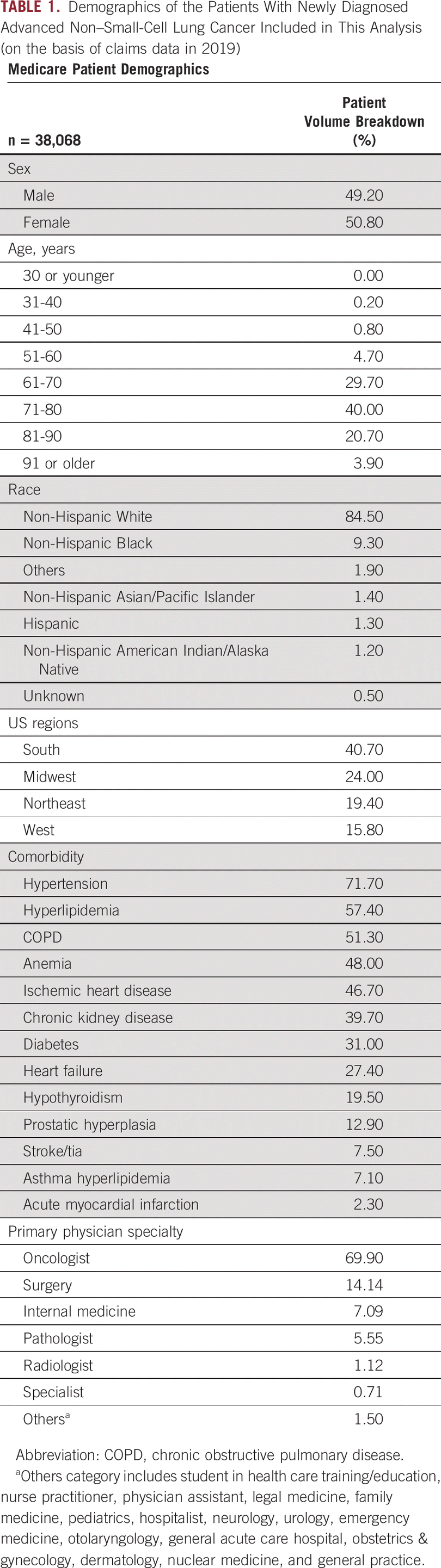
Demographics of the Patients With Newly Diagnosed Advanced Non-Small-Cell Lung Cancer Included in This Analysis (on the basis of claims data in 2019)
In the analyses of practice gaps 1 and 7, additional published data related to patients with aNSCLC statistics were used to confirm or supplement Diaceutics’ repository.14–16
In the analysis of practice gap 2, additional published data related to tissue and tumor cell sufficiency rates were used.17–19
In the analyses of practice gaps 3 and 6, additional published data were used related to methodology dependent analysis rates.20–25
In the analyses of practice gaps 5 and 7, additional published data related to methodology dependent FN, false-positive, or true-positive rates were used.15,20,22,25–35
Furthermore, in the analyses of practice gaps 5 and 7, additional third-party real-time laboratory data from US-based laboratories were used to supplement the Medicare claims data and laboratory test results data (eg, test performance and positivity rates) included in the Diaceutics repository.
A total of 5,589 patients with aNSCLC newly diagnosed in 2019 were identified within the real-time laboratory data set.
Although the direct overlap between the patients included in the two data sets was not determined, the data sets were correlated to make population-level assessments.
Data Supplement includes detailed information, broken down by practice gap, about how data from the Diaceutics Data Repository were combined with evidence from other sources to estimate the relative impact each gap contributes to the number of patients who could have but did not benefit from personalized medicine.

Clinical Practice Gap Analysis
The extent to which clinical practice gaps at each of the seven steps along the precision oncology pathway contributes to the overall number of patients who could have, but did not, benefit from receiving personalized medicine was quantified through an integrated approach using Diaceutics’ Data Repository along with additional sources of publicly available data as described above.
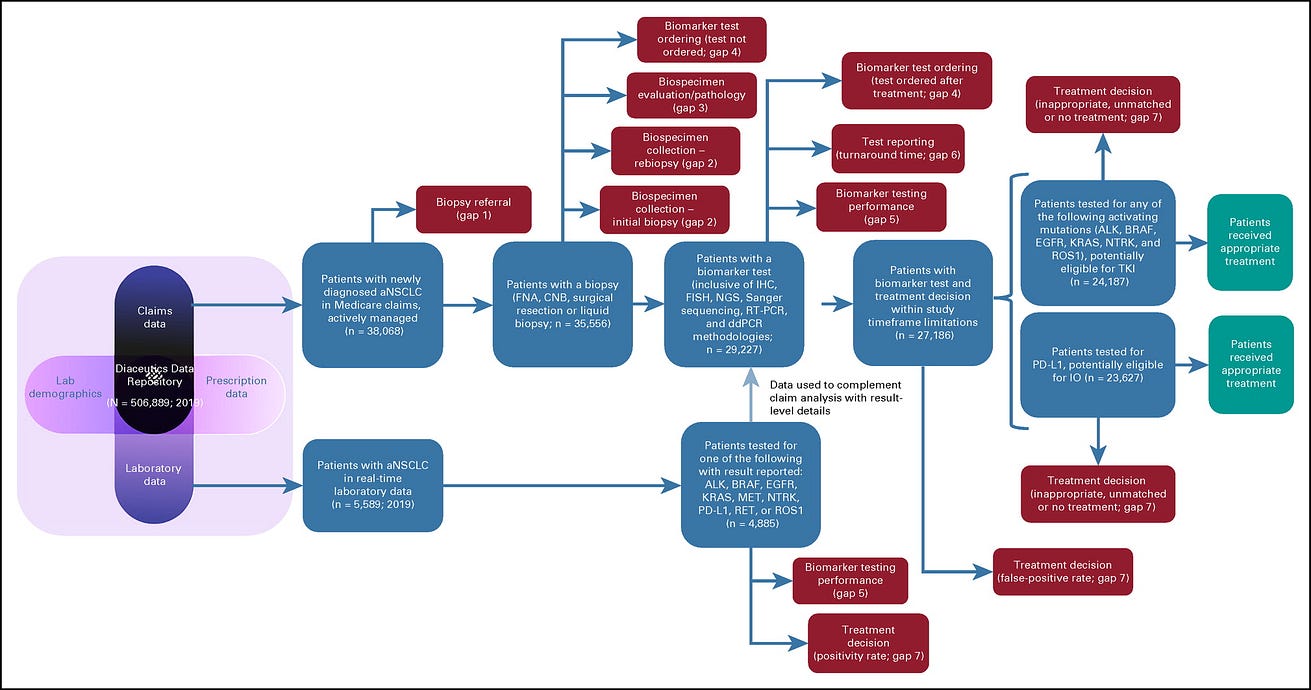
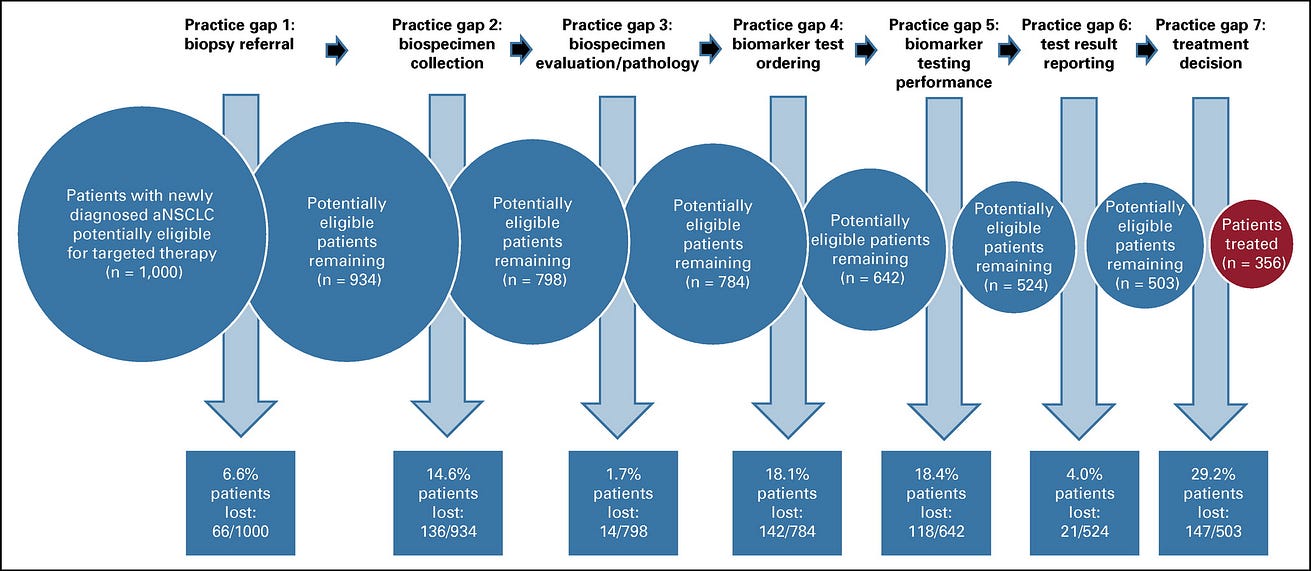
The step-by-step approach and the number of patients who were potentially available for analysis at each step of the journey are shown in Figure 1.
FIG 1. Step-by-step approach for analyzing the number of patients with advanced non–small-cell lung cancer lost at each step of the precision oncology pathway
Results
Data from 506,889 patients with NSCLC were included within the Diaceutics Data Repository in 2019.
Overall, 144,486 cases were first-time NSCLC insurance claims, indicating patients with newly diagnosed NSCLC. Of them, 38,068 were determined to be patients with actively managed aNSCLC. Of them, we evaluated the number and percentage of patients who advanced or were lost at each step within the clinical practice gap framework. We normalized the data to a patient population of 1,000 to easily demonstrate the percentage of eligible patients who may be lost to receiving targeted therapies because of each clinical practice gap ( Fig 2).
FIG 2. The precision oncology care pathway: Overall impact of clinical practice gaps on the loss of eligible patients to the delivery of personalized advanced NSCLC care.
CMS, Centers for Medicare & Medicaid Services; NSCLC, non–small-cell lung cancer; TAT, turnaround time; TNP, test not performed; QNS, quantity not sufficient.
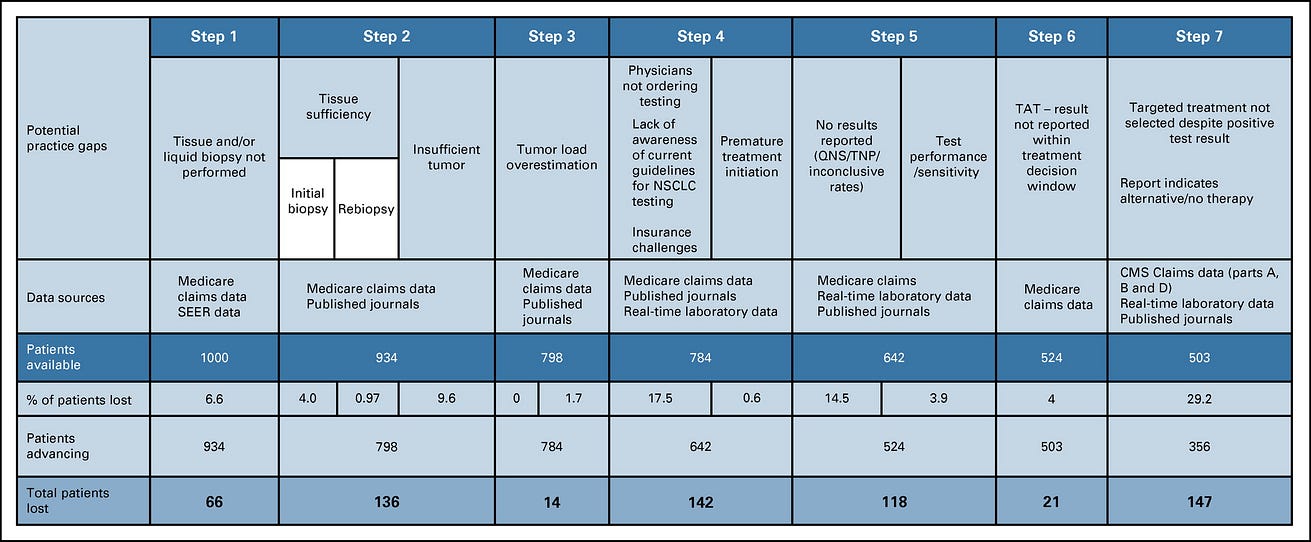
Clinical Practice Gap 1: Initial Biopsy Was Never Performed
Of the 38,068 patients with actively managed aNSCLC, our analysis reveals that 6.6% never had an initial biopsy performed (84.6% received a tissue biopsy; 8.8% received a liquid biopsy, 6.6% of patients without tissue or liquid biopsy were likely diagnosed through imaging only). Therefore, clinical practice gap 1 has led to 66 of 1,000 patients lost. The remaining 934 patients advance on the precision oncology care pathway.
Clinical Practice Gap 2: Biospecimen Collection Challenges Inhibited Biomarker Testing
Of the 32,224 patients with aNSCLC who received a tissue biopsy, data on the biopsy type were available for 9,425 cases, with 79.8% being conducted using fine needle aspirates (FNAs), 9.9% using core-needle biopsies (CNBs), and 10.3% involving surgical resections. We applied an insufficient tissue collected from biopsy rate of 4.5%17to the 89.7% of tissue biopsy samples including FNA plus CNB for an estimated 4.0% rate of patients with tissue insufficiency of initial biopsy to continue with biomarker testing. Liquid biopsies and surgical resections were assumed to have sufficient genetic material for biomarker testing.
Of the 9,425 patients who received a tissue biopsy of known type, 5.9% received a rebiopsy for various reasons. We applied an insufficient tissue rate for rebiopsy of 13.6%18to 2.7% of the overall samples (% of patients with FNA plus CNB rebiopsy) for an estimated 0.37% rate of patients who were unable to continue testing. Additionally, 0.6% of patients were lost who had original tissue biopsy collection problems but were not able to be rebiopsied for various reasons such as advanced disease progression or tumor inaccessibility and as a result did not go on to receive biomarker testing, resulting in a loss of a total of 0.97% of patients with tissue insufficiency of rebiopsy.
Of the patients with sufficient amount of tissue from biopsy or rebiopsy, an insufficient number of tumor cells available for testing within the tissue sample was estimated to be a problem for 10.7% of patients.19We applied the 10.7% rate of tumor cell unavailability to the 89.7% of samples obtained via FNA or CNB to determine a 9.6% rate of patients unable to receive testing because of the absence of sufficient tumor cells.
Thus, overall, practice gap 2 has led to 136 of 934 patients lost. The remaining 798 patients advance on the precision oncology care pathway.
Clinical Practice Gap 3: Biopsy Specimen Tumor Cell Content Was Overestimated, Inhibiting Biomarker Testing and Its Accuracy
Of the patients with aNSCLC who had adequate tumor biopsy samples, we estimate that 1.7% had tumor cell content that was overestimated. This was calculated as follows: 14% of tissues had < 20% tumor content and, therefore, may not meet the threshold requirements for specific testing platforms24(optimal tumor content is 10% for PCR-based tests, 20% for NGS-based tests, 30% for Sanger sequencing-based tests). We calculated that 38% of the 14% of tissue samples having < 20% tumor cell content were overestimated to have > 20% and were thus erroneously deemed appropriate to proceed for molecular testing, likely leading to inadequate testing results.23We applied a 38% overestimation rate to the 14% of tissues having < 20% tumor content to the share tested by NGS and Sanger and generated a potential patient loss rate of 1.7%. Practice gap 3 led to 14 of 798 patients lost. The remaining 784 patients advance on the precision oncology care pathway.
Clinical Practice Gap 4: Appropriate Testing Was Not Ordered or Treatment Began Before Testing Was Ordered
Of the 35,556 patients with aNSCLC who had either a tissue or liquid biopsy, 82.5% had biomarker testing ordered while 17.5% did not have any biomarker testing ordered. Many potential barriers may have affected test ordering for these patients. On the basis of an International Association for the Study of Lung Cancer (IASLC) survey (United States plus Canada data),36we estimate that of the 17.5% of patients with no tests ordered, 7.4% were lost because of cost concerns, 2.2% were lost because of test accessibility, 1.7% were lost because of a lack of awareness of testing options, 1.5% were lost because of low confidence in test value by the ordering physician or the patient, and 4.7% were lost because of other reasons.
On the basis of data from Medicare claims, 3.2% of patients received treatment before the ordering of biomarker testing. Of the patients who were quickly treated with immunotherapy, chemotherapy, or antivascular endothelial growth factor treatment, we estimate that 21.2% may have had an actionable biomarker, causing an additional 0.6% of eligible patients to be lost on the precision oncology care pathway.
Practice gap 4 has led to 142 of 784 patients lost. The remaining 642 patients advance on the precision oncology care pathway.
Clinical Practice Gap 5: Biomarker Testing Provided Inconclusive or FN Results
Of the patients who received biomarker testing, we estimate that 14.5% had an uninformative/inconclusive result because of several factors. On the basis of laboratory-level data, we show that of the 14.5% of uninformative results, 7.5% is due to technical failure leading to the test not being performed, 5.8% is due to sample quantity/quality not sufficient for testing that had not been detected during preanalytic processing, and 1.1% is due to inconclusive data (Data Supplement).
We applied test method-dependent sensitivity rates to patients who received a single molecular test to show the average level of FN rates for individual tests and to estimate the number of eligible patients lost because of FN rates (Data Supplement). Of the patients who received testing for actionable biomarker(s) and had a result reported, we estimate that 3.9% received a FN result. False positives are addressed as part of practice gap 7.
Practice gap 5 has led to 118 of 642 patients lost. The remaining 524 patients advance on the precision oncology care pathway.
Clinical Practice Gap 6: As a Result of Turnaround Time Delays, Treatment Was Initiated Without Consideration of Test Results
Of the 29,227 patients receiving biomarker testing with reported results, we estimate that 4% experienced laboratory TAT delays that led to treatment decisions that did not consider molecular testing insights. We analyzed the laboratory TAT capabilities of the top 30 aNSCLC US laboratories (representing 68.2% of aNSCLC market share) for each of the following methodologies: fluorescence in situ hybridization, IHC, NGS, and molecular (Sanger sequencing, ddPCR, and reverse transcriptase PCR). Differing testing platforms are associated with different TATs, but NGS testing was associated with the highest average TAT. We determined a 13.1% rate of problematic TAT by NGS (> 14 days as noted in guideline from the College of American Pathologists, IASLC, and the Association for Molecular Pathology)25for patients receiving results and applied this to the NGS-tested share for aNSCLC to estimate the number of patients who had treatment initiation before the return of testing results (Data Supplement).25
Practice gap 6 has led to 21 of 524 patients lost. The remaining 503 patients advance on the precision oncology care pathway.
Clinical Practice Gap 7: Targeted Treatment Was Not Selected Despite Positive Test Results
Of the 27,186 patients who received biomarker testing and received a timely result, we estimate that 29.2% did not receive the appropriate targeted treatment on the basis of their test results. On the basis of claims data, we determined that 18.5% of patients received no treatment. Reasons for a patient to not receive a treatment are presumed and may include patient death before the initiation of treatment, patient transferred to hospice care, or patient or caregiver electing to forego treatment. 81.5% of patients received various treatments, including 9.1% given chemotherapy only, 14.3% given chemotherapy and immunotherapy, 40.8% given immunotherapy only, 16.6% given targeted therapy, and 0.7% given other treatments.
Unmatched or inappropriate treatment after detection of an actionable mutation may occur for various reasons. We found that 14.3% of patients whose tumors were determined to have actionable results that determine targeted drug eligibility, such as for tyrosine kinase inhibitors, did not receive the indicated treatment. An additional 11.6% of patients with actionable results on the basis of IHC testing, such as to inform the use of antiprogrammed death-ligand 1 therapies, did not receive the appropriate immunotherapy. A total of 3.3% of patients were estimated to have incorrect test results (false positives) (Data Supplement).
The reasons for failure to act on positive test results were not determined in this analysis but can depend on various barriers related to receiving targeted therapies, such as reporting issues (errors in reports, outdated clinical information, and missing drugs); lack of US Food and Drug Administration-approved indication (physician unclear or unwilling to use off-label); and lagging awareness of targeted treatment options and/or guidance, social determinants of health access/disparities (therapy accessibility constraints); therapy cost/insurance coverage concerns; or comorbidities/contraindications ( Fig 3).
FIG 3. Barriers to receiving biomarker-based therapy for patients with actionable mutations
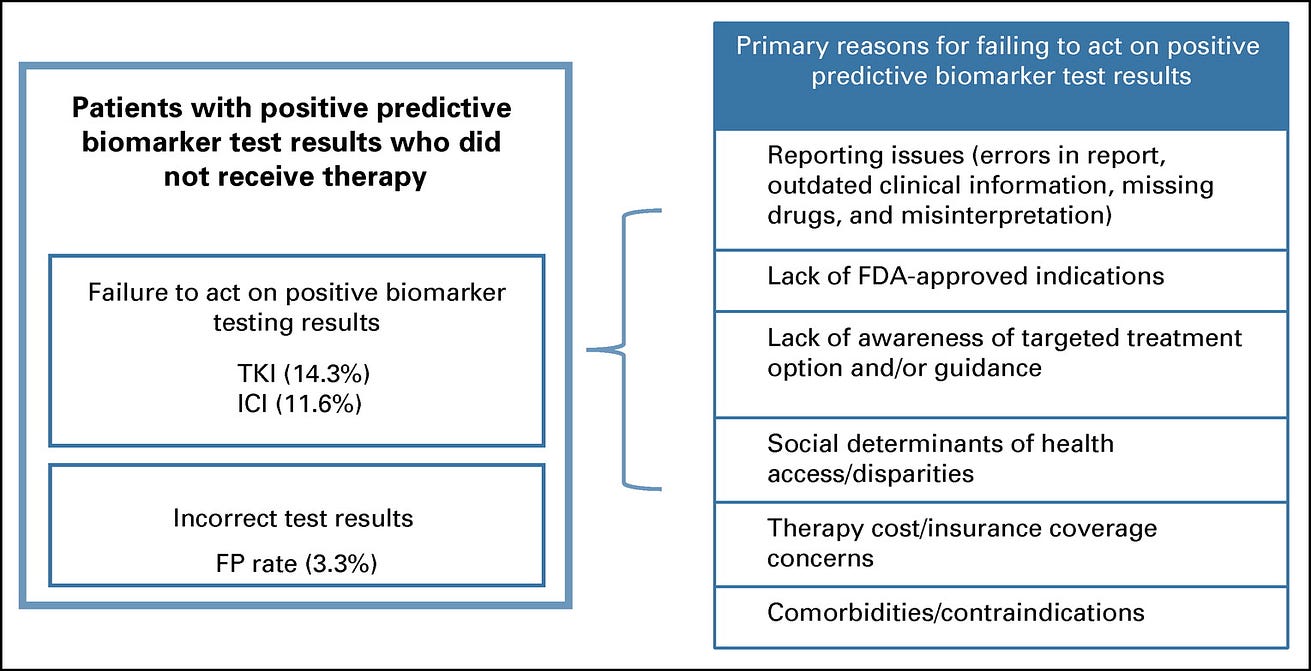
Practice gap 7 has led to 147 of 503 patients lost. The remaining 356 patients advance on the precision oncology care pathway.

Results Summary
Overall, we estimate that for every 1,000 patients with newly diagnosed aNSCLC who are potentially eligible for targeted therapy, a cumulative tally of 497 are lost to factors associated with getting a biomarker test while 147 biomarker-positive patients are not prescribed the appropriate targeted therapy ( Fig 4).
FIG 4. Impact of clinical practice gaps on the delivery of precision oncology for aNSCLC
Thus, 644 of every 1,000 patients with newly diagnosed aNSCLC (64.4%) are not benefiting from precision oncology care options appropriate for their diseases and will likely have suboptimal outcomes.

Discussion
We found that only 356 of 1,000 (approximately 36% of) eligible patients with aNSCLC are benefitting from precision oncology treatments appropriate for their condition.
Our results show that 497 of 1,000 (approximately 50% of) patients are lost along the precision oncology pathway because of preanalytic and analytical practice gaps related to biospecimen processing or diagnostic test ordering, performance, and reporting.
Of the 503 of 1,000 patients who do get biomarker testing and have reported actionable mutations, approximately 147 (29.2%) are lost because of post-testing practice gaps.
Although the relative impact of each clinical practice gap will vary across health care delivery institutions, our estimates provide baseline indications for the overall US health care system.
The findings convey a sense of the magnitude of the challenge associated with each practice gap, which can help catalyze and inform strategies to address them.
Our results generally concur with several previously published studies examining biosample processing efficiency and the delivery of personalized medicine, …
… including studies that showed variable testing rates for different cancers3,5and rates of patients who test positive for actionable biomarkers but do not receive targeted therapies.8,9
In our evaluation of practice gap 1, the number of lost patients who never received an initial biopsy corroborates with NSCLC data from the SEER Program on cancer statistics.14
Overestimation of tumor content load (evaluated in practice gap 3) can lead to FN results for Sanger sequencing- and NGS-based tests.
However, this is not the only factor driving FN results.
In our evaluation of practice gap 5, we focused mainly on FN rates driven by causes other than tumor load overestimation and expanded our analysis to all technologies, leaving minimal overlap between these two analyses.
It is important to keep in mind that although the different gap analyses are shown as discrete steps, they are all connected and influence the overall delivery of care.
Here, we combine data reflecting all of the clinical practice gaps to clearly show the magnitude of the issues and to highlight that more patients than previously expected are actually not receiving the most appropriate treatment.
Our estimations are subject to various limitations.
We have only analyzed patients with procedures and treatments that are included within claims data cross-referenced to laboratory data, so patients who are enrolled in clinical trials who receive procedures that are not represented in a claim would not be included.
Although we used practice-based data from the Diaceutics repository whenever possible, we relied on published data to supplement the 2019 repository data, sometimes from sources that include data from earlier time periods.
Although we have used the most recent and relevant data available to determine the current impact of clinical practice gaps, some of the older published data may not reflect current evolving practice standards.
The analysis of practice gap 4, biomarker test ordering, was based on claims data from the Diaceutics Data Repository, but evaluation of factors that can lead to a test not being ordered was based on US and Canada IASLC data.
However, even with survey data providing reasons why treating physicians did not order a test for their patients, the true driver or combination of drivers is complex and remains subject to estimation.
When evaluating test performance rates, we calculated average positive testing rates using laboratory data within the Diaceutics Data Repository and data from the American Association for Cancer Research’s Genomics Evidence Neoplasia Information Exchange project (an international pan-cancer registry of real-world data)15weighted by the number of patients tested by laboratories represented in both databases.
Although this strategy is meant to allow for the largest possible statistical sampling to drive the determination of average positivity rates, actual rates may differ depending on the patient cohort.
When evaluating the number of patients lost to precision oncology because of TAT, we based our evaluation on laboratory TAT only, while, in fact, total TAT can be longer because of multiple reasons and can affect more patients.
Although causes of practice gaps related to test ordering, processing, and performance are relatively clear, the causes of downstream gaps are less clear.
Patients who receive suboptimal treatment because of misinterpretation of test results are not differentiated from patients who had appropriate interpretation of test results but still did not get treatment matched to their results.
Physicians may fail to act on positive biomarker test results because of a number of factors ( Fig 3). The relative impact of each of these causes is not clear.
Nonetheless, these issues have been identified as barriers to the delivery of personalized treatment,3,37,38and efforts to address each of these challenges should incentivize or improve the use of biomarker test results to deliver appropriate targeted therapies and reduce the overall impact of this practice gap.
Our analysis considers precision oncology biomarkers including genomic variants that can inform targeted therapeutic options and immune checkpoint inhibitors that can be used to inform immunotherapeutic options.
However, we have separated out IHC-based testing for immune checkpoint inhibitors from genetic testing to help show the relative impact on the clinical practice gaps of each of these biomarker types.

Conclusion
In conclusion, this analysis leverages claims and laboratory data to provide real-world evidence demonstrating the impact of various clinical practice gaps on the delivery of precision oncology care.
Only approximately 36% of patients with aNSCLC are benefiting from precision oncology, indicating a significant clinical impact deficit.
This finding warrants further investigation, investment, and action.
Decision makers would do well to consider the impact of each practice gap when prioritizing and developing process and practice standards designed to improve the delivery of clinical care.
Although this study highlights the need for efforts to address all practice gaps and to develop precision oncology implementation improvement strategies, it represents a snapshot in time using data from 2019.
These results should be updated over time to gauge progress and to show the impact of improved delivery efforts.
Although these findings are made in aNSCLC, they are likely reflective of similar gaps in other cancer types, indicating a need for further investigation and action across oncology practices.
Although these findings are made in aNSCLC, they are likely reflective of similar gaps in other cancer types, indicating a need for further investigation and action across oncology practices.
Addressing practice gaps can lead to improved clinical care associated with a precision oncology approach.
Attention to practice gaps may also help to decrease health care costs through enhanced systemic efficiency and potentially reduced downstream spending on hospitalizations and health resource expenditures necessitated by suboptimal earlier care.
An increased understanding of the impact of practice gaps can thus inform strategies to deliver more fully on the promise of personalized medicine.
Addressing practice gaps can lead to improved clinical care associated with a precision oncology approach.
Attention to practice gaps may also help to decrease health care costs through enhanced systemic efficiency and potentially reduced downstream spending on hospitalizations and health resource expenditures necessitated by suboptimal earlier care.
References and additional information
See original publication.
Originally published at https://ascopubs.org.
About the authors & affiliations
Helen Sadik, PhD1;
Daryl Pritchard , PhD2;
Derry-Mae Keeling, BSc1;
Frank Policht, PhD1;
Peter Riccelli, PhD1;
Gretta Stone, BS3;
Kira Finkel, MSPH3;
Jeff Schreier, MBA1; and
Susanne Munksted, MS1
1 Diaceutics, Belfast, United Kingdom
2 Personalized Medicine Coalition, Washington, DC
3 Reservoir Communications Group, Washington, DC
Support
Supported in part by the Personalized Medicine Coalition, a nonprofit 501c3 organization dedicated to the advancement of personalized medicine. H.S.:
Thermo Fisher Scientific, AstraZeneca, Eli Lilly and Company, Blueprint Medicines, Oncocyte.












