health transformation institute (HTI)
the most comprehensive knowlege portal, for healh transformation
Joaquim Cardoso MSc*
Founder and Chief Researcher and Officer
November 26, 2022
MSc* from London Business School — MIT Sloan Masters Program
This is a republication of the article “Better brain biology will deliver better medicines”, with the title above.
The Economist
Sep 21st 2022
A broken brain, hidden inside a skull, is harder to diagnose than a broken leg.
The fact that there is somebody inside the skull to tell doctors how they feel might seem to offer a way round this.
But the feelings patients describe are not easily mapped on to the brain.
Definitions of mental diseases are vague. “Major depressive disorder and generalised anxiety have an 80% overlap in disease definitions,” says Daniel Karlin, the chief medical officer of MindMed, a biotech firm.
Definitions of mental diseases are vague. “Major depressive disorder and generalised anxiety have an 80% overlap in disease definitions,” …
What patients say they are feeling is also a possible source of confusion.
Amit Etkin, the ceo of Alto Neuroscience, points out that in Asia the symptoms that Western psychiatry uses to diagnose depression-low mood, tearfulness and the like-do not work very well, because the symptoms are expressed in more physical terms:
“‘I feel cold,’ ‘My limbs are heavy’, or abdominal distress”. But when depression is defined at a biological level, Dr Etkin says, the same distinct subtypes emerge-three to five of them, he reckons.
Alto is trying to use eeg s and behavioural testing to match patients to drugs to improve the chances of giving patients the treatments that work best for them.
Alto is trying to use eeg s and behavioural testing to match patients to drugs to improve the chances of giving patients the treatments that work best for them.
There are other arguments for taking issue with established diagnostic procedures.
Take autism. A study published in 2021 found between 1998 and 2018 there had been a 787% increase in diagnosis in Britain. In America, it is now diagnosed in one in 44 children.
Uta Frith, a professor emerita of cognitive development at University College London who worked on autism throughout that time, said this was evidence that the diagnosis had been “stretched to breaking point and has outgrown its purpose”.
The experiences of people affected by attention-deficit hyperactivity disorder ( adhd), depression, Parkinson’s and other conditions also vary immensely.
There is a growing awareness among those looking for therapies that better, more biological ways of defining patient populations are needed; better psychiatric treatments require clearer ideas about what needs fixing in whom.
There is a growing awareness among those looking for therapies that better, more biological ways of defining patient populations are needed; better psychiatric treatments require clearer ideas about what needs fixing in whom.
“I don’t think in 20 years we will be calling things ‘Alzheimer’s’,” Ms Bingham of sv Health Investors predicts. “I think we will be talking about diseases driven by specific pathways and biologies.”
“I don’t think in 20 years we will be calling things ‘Alzheimer’s’,” Ms Bingham of sv Health Investors predicts. “I think we will be talking about diseases driven by specific pathways and biologies.”
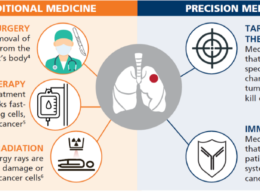
The model here is oncology.
Cancers used to be treated according to the organ in which they were found and their morphology.
Today oncology is moving quickly towards molecular precision, identifying the specific pathway or pathways that are driving a cancer’s growth and attacking them directly with a range of tools from small molecules to antibodies to genetically engineered immune cells.
When Dr Etkin argues that the biology driving depression should be more “measurable, conserved and objective” he is speaking for a generation of researchers who want a similar level of insight into the mechanisms of the mind.
Today oncology is moving quickly towards molecular precision, identifying the specific pathway or pathways that are driving a cancer’s growth and attacking them directly with a range of tools from small molecules to antibodies to genetically engineered immune cells.
One advantage such measurements offer is the ability to “stratify” patients-that is, to split them into groups of patients who differ in some biologically relevant way.
In September, scientists from Rensselaer Polytechnic Institute, in New York, proposed that the autism spectrum could be split into three distinct categories associated with different maternal risk factors such as infections, joint disorders and pregnancy complications.
These sorts of findings allow distinct patient groups to be identified, making trials more likely to be clear cut.
In September, scientists from Rensselaer Polytechnic Institute, in New York, proposed that the autism spectrum could be split into three distinct categories associated with different maternal risk factors such as infections, joint disorders and pregnancy complications.
Neumora, a remarkably well-funded startup based in Watertown, Massachusetts, puts “deconvolving” patient populations into more and more homogenous subtypes at the core of its work.
As Paul Berns, head of the firm and of arch, a venture-capital firm which is one of Neumora’s investors, puts it, “We can’t treat everybody the same way. We are getting really poor outcomes and spending a lot of money.”
The firm is making use of data on large cohorts of people, such as those collected by uk Biobank, which is following half a million people over decades, and the Parkinson’s Progressive Markers Initiative funded by the Michael J. Fox Foundation.
These cohorts show how diseases change over time at varying levels, from the genes on up.
… “We can’t treat everybody the same way. We are getting really poor outcomes and spending a lot of money.”
The firm is making use of data on large cohorts of people, such as those collected by uk Biobank, which is following half a million people over decades, and the Parkinson’s Progressive Markers Initiative funded by the Michael J. Fox Foundation.
One of Neumora’s projects is a trial for a drug which blocks the kappa opioid receptor ( kor) in patients with severe depression.
The trial is focused on patients with a high score for “anhedonia”, the inability to feel pleasure.
John Dunlop, Neumora’s head of r& d, says data suggest that the kor is expressed in areas of the brain that deal with motivation and reward.
So if the drug works well in depressed patients with anhedonia it might be useful in other diseases where the deficit also crops up, such as schizophrenia and post-traumatic stress disorder.
Similar approaches based on mechanism, rather than a classic diagnosis, have served oncology well, producing drugs such as pd-1- and parp-inhibitors that cross organ-based cancer categories.
Whether anhedonia cleaves closely enough to a detailed, and correctable, molecular mechanism in the brain will only become clear with further work.
One of Neumora’s projects is a trial for a drug which blocks the kappa opioid receptor ( kor) in patients with severe depression. The trial is focused on patients with a high score for “anhedonia”, the inability to feel pleasure.

Good for what ails you
Other approaches look into the basic biology of the brain’s development.
In 2006 a technique was discovered whereby body cells could be turned into stem cells able to develop into a range of specialised cell types.
With the right encouragement, and a three-dimensional scaffold on which to grow, neural stem cells made this way give rise to complex “organoids” made up of a range of different types of neuron and some glial cells, too.
These “mini brains” composed of human cells have opened a whole new field of research, making it possible to compare organoids from people who suffer from an affliction with those of people who do not.
Organoids grown from cells from people with autism, for example, have more “inhibitory” neurons than is typical, which may make them prone to develop particular types of neural circuitry.
Another distinctively 21st-century approach is optogenetics.
By adding genes for fluorescent proteins to an animal’s genome it is possible to see different pathways in the brain light up as they are activated.
More remarkably, by adding novel genes which make proteins on cell surfaces light-sensitive, it is possible to create brains where the behaviour of specific types of neuron can be controlled from outside by light.
Another distinctively 21st-century approach is optogenetics. By adding genes for fluorescent proteins to an animal’s genome it is possible to see different pathways in the brain light up as they are activated.
Lab animals grown with such modifications can show how, at a neurological level, they regulate complex behavioural states.
Fluorescent markers reveal how neurons connect to each other, and allow a map of the brain’s highways and byways to be created-a “brainbow”.
This has shown how patterns of neural activity regulate functions such as thirst, respiration, energy balance and sleep.
It has revealed the dynamics of information transmissions and the patterns of activity that are involved in some of the brain’s plasticity.
Lab animals are not the only targets for genetic modification. Some patients undergo it too, in the form of gene therapies.
In 2019, the gene-therapy drug Zolgensma was approved for use in patients with spinal muscular atrophy ( sma)-a progressive condition in which the loss of motor neurons weakens muscles.
It is caused by mutations in the gene without which motor neurons find it hard to survive.
Zolgensma delivers competent copies of the smn1 gene to nerve cells inside a viral “vector”.

On your biomarkers
Similar gene therapies are also a promising approach to various diseases of the retina-the sheet of light-sensitive cells and neurons at the back of the eye-and to Dravet syndrome, a form of epilepsy.
Lysosomal storage disorders, inherited metabolic diseases that are the source of many neurodevelopmental problems, also look like promising gene-therapy targets.
But some diseases caused by an errant gene are proving challenging to address with this method.
Efforts to tackle Huntington’s, a fatal neurological disorder, with gene therapy have been fraught with difficulties.
Similar gene therapies are also a promising approach to various diseases of the retina-the sheet of light-sensitive cells and neurons at the back of the eye-and to Dravet syndrome, a form of epilepsy.
Gene therapy is not the only approach to inherited disorders traced to a single gene.
There are various cunning ways short pieces of dna and its relative, rna, can be used to change the amount of protein made according to the recipe in a particular gene, either reducing it or, sometimes, increasing it.
Ionis Pharmaceuticals is working with Biogen to expand the use of some of these approaches in neurological diseases.
It is testing a drug called tofersen to treat a form of als caused by a problem with the s od1 gene-the defect behind the disease in 2% of patients.
It is a good example of the benefits of stratification; if all forms of als were seen as the same, the potential of a drug that addresses a specific fraction of them would go unnoticed.
The tofersen trial is also a good example of the potential of biomarkers.
A measurable biomarker that is well correlated to the course of disease gives researchers an early peek at whether success seems likely.
The trial has seen “robust” reductions in the level of neurofilament (the structural components of the sheath that insulates nerve axons) in the blood.
When neurons are injured, proteins from these structures are released into the blood.
Their level is thought to indicate the numbers of damaged or degenerating nerves.
In multiple sclerosis levels of neurofilament are lowered by treatment with a number of new disease-modifying therapies.
The emergence of neurofilament, and other biomarkers, as trusted signs of the underlying course of a disease should facilitate a flurry of innovation.
The emergence of neurofilament, and other biomarkers, as trusted signs of the underlying course of a disease should facilitate a flurry of innovation.
The blood is not the only place to look for biomarkers.
Imaging instruments and the precise study of bodily behaviour can also furnish them.
But those found in the blood are particularly useful because of the ubiquity of blood testing.
Biomarkers that truly track the course of a disease are not just useful for doctors and clinical trials.
Some might also provide early warning of its development before the onset of symptoms-in time, perhaps, to make changes to the way in which they live.
The blood is not the only place to look for biomarkers. Imaging instruments and the precise study of bodily behaviour can also furnish them.

TREMendous stuff
The usefulness of such changes in patients’ behaviour and situation is a reminder that, in most diseases of the brain, genes are only one factor, and a complicated one at that.
The most common diseases of the brain are influenced by factors such as diet, exercise, the environment, life history and other diseases as well as by a set of genes the membership of which has risen rapidly as the sequencing of whole genomes has accelerated.
There are now more than 100 genes associated with Alzheimer’s, Parkinson’s and als.
Denali, a biotech company in San Francisco which is at the forefront of the field, calls genes involved in neurodegeneration “degenogenes”, echoing the use of “oncogenes” in studies of cancer.
Identifying such genes is potentially helpful; it has also at times been misleading.
Early genetic studies provided some evidence for a connection between Alzheimer’s and beta amyloid which, in concert with the role of amyloid plaques in the disease, encouraged drugmakers down a blind alley.
One school of thought is that genetic studies of people who have been diagnosed with a disease may be finding genes that have failed to protect their nerve cells from the progression of disease, rather than genes that identify the disease’s causal mechanism.
In Alzheimer’s, the broader range of associated genes now on offer may provide new clues.
Sabah Oney, a venture partner with arch, says that if one looks at genes for the root cause of Alzheimer’s it is startling that 22 of the 25 highest-risk genes map directly to the immune system-and specifically to its inactivity.
He likens the brain’s immune system to the fire brigade, police and rubbish collection. They all need to be continuously active to keep the brain healthy. If any fails, pathological damage accumulates.
… if one looks at genes for the root cause of Alzheimer’s it is startling that 22 of the 25 highest-risk genes map directly to the immune system-and specifically to its inactivity.
One focus in the study of dementia is trem2, a gene that codes for a protein on the surface of the microglia involved in the brain’s bit of the immune system.
Alector, a biotech firm based in San Francisco, is one of the companies that think a drug aimed at that receptor protein might boost the microglia’s activity; it is developing an antibody drug to that end.
Again, there is an analogy to oncology, where getting the immune system better engaged in the fight against disease is the focus of much research.
Alector’s work has drawn the attention of gsk, a big pharma firm, which invested $700m last year.
Alector, a biotech firm based in San Francisco, is one of the companies that think a drug aimed at that receptor protein might boost the microglia’s activity; it is developing an antibody drug to that end. Alector’s work has drawn the attention of gsk, a big pharma firm, which invested $700m last year.
Vigil Neuroscience, based in Massachusetts, is also targeting trem 2 as part of a strategy focused on microglia.
Investment in startups like Neumora, which explicitly brands itself a precision-neuroscience company, and gene-inspired pathway-specific approaches like those of Vigil and Alector, show that this approach is becoming popular with both researchers and investors.
But not everyone is convinced.
“Precision neurology is a bit of an oxymoron from a pharmacological perspective,” says Duncan Emerton of Citeline Pharma Intelligence, a data provider.
“Current treatments for neurological indications are very rarely precise in their mechanism of action, with numerous off-target effects being seen.” It may come to be, but it is not here yet.
But not everyone is convinced. … some believe that so far the concept is incomplete.
Jeff Jonas, chief innovation officer at Sage Therapeutics, a drug company, believes that so far the concept is incomplete.
“The idea that you can find a single target that will give you an efficient pathway-it just has never been shown to be the case”.
He likens the effort to precision elephant-recognition, which provides good data on tails and trunks, but limited understanding of the animal.
Sage’s approach is to look for drugs active in the brain and seek out big effects. Its drug zuranolone, a neuroactive steroid, is related to a steroid already used to treat postpartum depression.
Dr Jonas says it resets the brain’s normal balance and, as a result, changes the end state of depression. The drug is intended to act quickly, far faster than traditional antidepressant drugs.
Whoever is right about the best approach to precision neuroscience, biomarkers and more tightly defined patient groups is certainly likely to yield stronger signals about the efficacy of trials-something that will improve the disastrous economics of the field.
And in many ways, disagreement in the field about the right approach is to be welcomed.
The failures of the past came from too much groupthink and a focus on the same narrow idea.
A thousand flowers are blooming. Some of them will wither and die.
But with so many new biotechs digging deep where big pharma has feared to tread, progress by some of them is inevitable.
A thousand flowers are blooming. Some of them will wither and die.
But with so many new biotechs digging deep where big pharma has feared to tread, progress by some of them is inevitable.
Originally published at https://www.economist.com on September 21, 2022.
Names mentioned
Amit Etkin, the ceo of Alto Neuroscience,
Daniel Karlin, the chief medical officer of MindMed, a biotech firm.
Uta Frith, a professor emerita of cognitive development at University College London
John Dunlop, Neumora’s head of r& d,
Jeff Jonas, chief innovation officer at Sage Therapeutics
Sabah Oney, a venture partner with arch,
Duncan Emerton of Citeline Pharma Intelligence, a data provider.