Also: Truepill launches a COVID-19 test coverage platform for insurers, and 23andMe extends its partnership with GSK.
Mobile Health News
By Emily Olsen
January 20, 2022
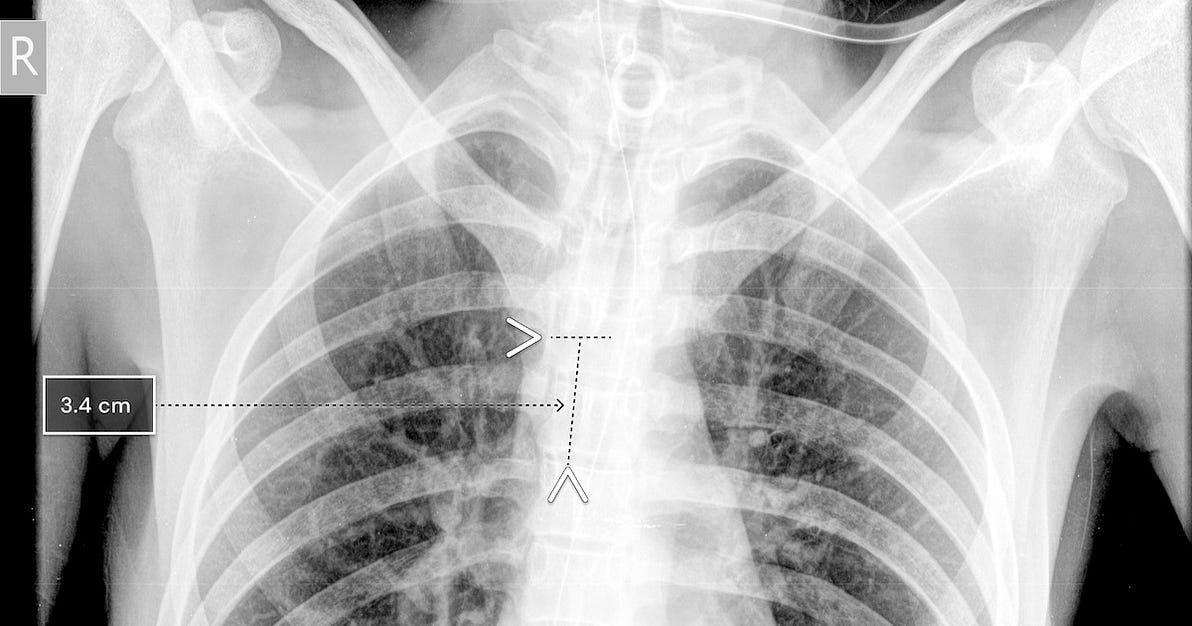
Qure.AI scored FDA 510(k) clearance for its artificial intelligence algorithm that assists providers in placing breathing tubes.
The clearance, which officially came down in late December, is the company’s third 510(k). The two previous FDA green lights were regarding its brain CT scan product.
Using chest x-rays, the qXR-BT algorithm can analyze the position of the breathing tube. It automates measurement and can report accuracy to the clinician, who can then decide if the tube needs to be re-placed.
“
We are pleased to have received FDA clearance for qXR-BT. In the last two years, we have seen the need to decrease processing times and solve workflow delays,” cofounder and CEO Prashant Warier said in a statement. “Especially in the wake of the COVID-19 pandemic and the need for mechanical ventilation in affected patients, the need for prompt assistance to an overburdened healthcare workforce is paramount.”
Digital health platform Truepill launched a COVID-19 test coverage platform to assist insurers that are now required to cover the costs of over-the-counter, at-home tests.
That platform will allow payers to develop direct-to-consumer test purchasing sites for their members, with Truepill fulfilling and shipping orders.
The digital health company has also launched a COVID-19-focused virtual care platform and a suite of diagnostics and digital tools for the employer market.
In October, it announced it had raised $142 million in Series D funding.
Consumer genetics company 23andMe and pharma giant GlaxoSmithKline are extending their drug discovery collaboration until July 2023.
The partnership, which began in 2018 when GSK invested $300 million in the genetics startup, will net 23andMe a one-time payment of $50 million to extend the partnership period.
The companies also announced 23andMe is taking a royalty option on their joint immuno-oncology antibody program, which is currently in Phase 1 studies. GSK will solely handle the rest of development and later-stage trials.
“The collaboration with GSK has been very productive. In less than four years, under this collaboration, we have identified over 40 therapeutic programs and have advanced an immuno-oncology antibody targeting CD96 into clinical development,” Kenneth Hillan, head of therapeutics at 23andMe, said in a statement.
“GSK’s decision to extend the exclusive target discovery period of our collaboration for an additional year demonstrates the enthusiasm for our collaboration and the value our database provides for identifying targets and advancing new medicines based on human genetics.”
23andMe went public last year through a merger with a special purpose acquisition company.
In October, it dove deeper into healthcare when it announced plans to acquire telehealth and online prescription platform Lemonaid Health.
Earlier this month, it earned FDA 510(k) clearance for a genetic test to detect a hereditary marker for prostate cancer.
Originally published at https://www.mobihealthnews.com on January 20, 2022.












