the health strategist
knowledge platform
health management, engineering and technology
for continuous transformation
Joaquim Cardoso MSc.
Chief Research and Strategy Officer (CRSO);
Chief Editor and Senior Advisor
August 3, 2023
What is the message?
The medical device industry is embracing AI and machine learning, with a focus on radiology and cardiology, leading to increased FDA authorizations and the need for adaptive regulatory approaches.”
What are the key takeaways?
There is a Surge in AI-Enabled Medical Devices:
- The number of AI- and machine-learning-enabled medical devices authorized by the FDA has been increasing rapidly over the past few years.
- In 2022 alone, the FDA authorized 91 such devices, showing a significant growth trend.
The concentration is in Radiology and Cardiology:
- Most of the AI devices have been developed for radiology, followed by cardiology.
- These specialties have larger and cleaner datasets, making it easier for developers to create effective algorithms.
Regulatory approval does not require clinical trials
- The majority of AI and ML medical devices have received 510(k) clearance, which does not require clinical trials.
- Only a few devices have gone through the more rigorous premarket approval process.
There are 2 dominant players:
- GE Healthcare and Siemens Healthineers have the most authorized AI/ML medical devices.
- GE Healthcare has focused primarily on radiology, while Siemens Healthineers has a wide range of cleared devices.
Devices are expected to increasing in complexity:
- AI/ML devices are expected to become more complex, with algorithms that can “learn” and adapt over time.
- The FDA is working on modernizing its regulatory approach to accommodate these dynamic algorithms.
The is a need for collaboration with Clinicians:
- Developers need to involve clinicians in the early stages of design to ensure the software is effective when integrated into medical practice.
DEEP DIVE

MedTech Dive Trendline on Medical device industry continues to turn to AI
As medical devices that use artificial intelligence and machine learning appear in more hospitals and imaging labs across the U.S., new data from the Food and Drug Administration show the agency has been fielding more submissions.
In 2022 alone, the FDA authorized 91 AI- or machine-learning-enabled medical devices, according to data released on Oct. 5. This broad category of devices can include anything from a basic algorithm to more complex machine learning tools, Michaela Miller, U.S. medtech technology and analytics practice leader for IQVIA, a North Carolina-based analytics and clinical research firm, said by email.
Devices that received FDA clearance this year include an atrial fibrillation history feature for the Apple Watch, and Tel Aviv-based startup Aidoc’s feature to analyze X-ray exams to detect and triage collapsed lungs.
Dive analyzed FDA data on all of the AI- and machine-learning-enabled devices the agency has authorized to date. Here are five takeaways on the rise of these devices.
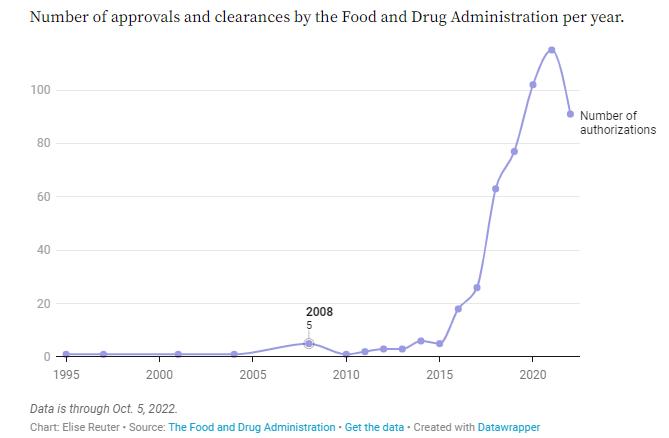
1. The number of AI-enabled medical devices has surged in the last five years
The number of AI- and machine-learning-enabled devices reviewed by the agency more than doubled from 2017 to 2018, and has continued to grow each year since. In 2021, the FDA authorized a record 115 submissions, an 83% increase from 2018.
Advances in sensor technology, imaging and data analytics have helped drive the trend, Miller wrote. Still, “the need to regulate and control these technology applications as drivers behind treatment decisions has increased to ensure patient safety,” she added.
Just two years ago, the FDA created its Digital Health Center of Excellence, adding staff to provide more expertise on software devices and to modernize the agency’s regulatory approach. Bakul Patel, who led that initiative, has since left the agency, but while there Patel built significant capacity to evaluate AI- and machine-learning-enabled devices, said Nicholson Price, a professor of law at the University of Michigan and who focuses on life sciences innovation.
“
There have been questions about capacity constraints on FDA, whether they have the staff and relevant expertise,” Price said. “They had a plan to increase hiring in this space, and they have in fact hired a bunch more people in the digital health space.”
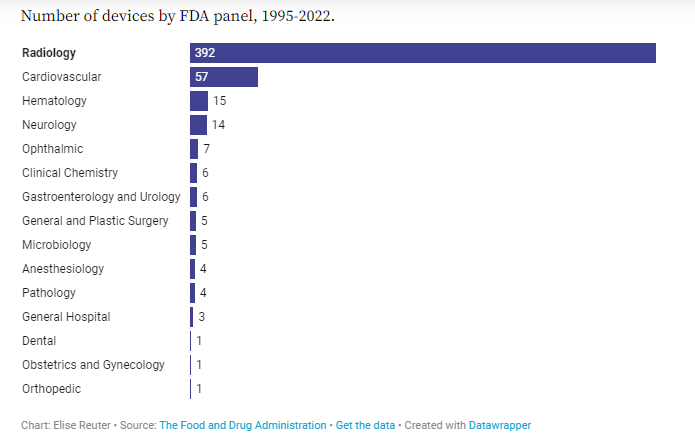
2. There are more AI tools for radiology than for any other specialty
Of the 521 submissions the FDA has authorized to date, three-quarters have been in radiology and 11% have been in cardiology. Part of the reason for the concentration of devices in these specialties is because there is ample data for device developers to draw on from imaging and electrocardiograms.
“
Radiology went digital very early relative to other areas of medical practice,” Price said. “You’re not working with as messy of [data], different sources of text analysis, differences in how things are measured. … You’ve got bigger, more comparable, cleaner datasets in [radiology and cardiology] than you have in a lot of other areas.”
Because data in other areas often isn’t configured the same way across most hospitals and healthcare systems, an algorithm that uses data pulled from patients’ health records to make a prediction at one hospital may not work in another health system, Price said. Even if both hospitals use the same electronic health record system, differences in how that system is set up can affect how well the algorithm works.
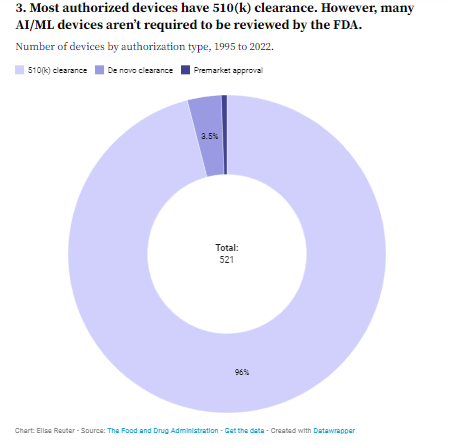
3. Most authorized devices have 510(k) clearance. However, many AI/ML devices aren’t required to be reviewed by the FDA.
Of the devices that have gone through FDA review, the majority have received 510(k) clearance, which does not require clinical trials as long as developers can prove their device is “substantially equivalent” to one already on the market.
To date, 96% of authorized AI- and machine-learning-enabled medical devices have 510(k) clearance, while only three devices have gone through the FDA’s more rigorous premarket approval process. Another 18 devices have gone through the regulator’s de novo clearance process, which is for devices that are not deemed high risk but do not have a predicate.
While most of the devices on the market don’t provide a diagnosis, they instead offer suggestions or alerts for clinicians or patients.
For example, detecting atrial fibrillation from ECG data may be a relatively simple task from an analytical perspective, but it won’t have the scope of information that a clinician uses when they make a diagnosis, said Giorgio Quer, assistant professor of digital medicine and director of artificial intelligence at the Scripps Research Translational Institute.
“
Devices are focused on a specific task. They are fantastic at that task,” he said. “My device, for example, is telling me, ‘you’re probably ok’ or ‘you may have an arrhythmia, let’s get it checked.’ It cannot tell you, ‘you have exactly this type of arrhythmia.’ This is the task of a clinician to interpret the data.”
FDA-authorized devices likely are just a fraction of the AI- and machine-learning-enabled tools that exist in healthcare as most applications of automated learning tools don’t require regulatory review, according to Price. Academic medical systems generally haven’t been required to seek FDA authorization for algorithms they develop in-house, Price added. Predictive tools based on electronic health record systems were exempted from regulatory review, though that may change under a new guidance.
“
My strong impression is that somewhere between the majority and vast majority of ML and AI systems being used in healthcare today have not seen FDA review,” Price said. “It sure seems like medical devices that are going to be impacting health and safety should go through some sort of regulatory review. Exactly what that should look like is fairly complicated.”
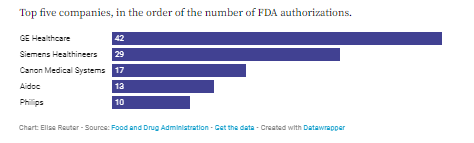
4. GE Healthcare and Siemens have the most authorized AI/ML medical devices
So far, GE Healthcare has received FDA clearance for 42 AI- and machine-learning-enabled medical devices, most of which are in radiology. They include an image-reconstruction algorithm to improve the quality of MRI images and an algorithm to help clinicians detect collapsed lung cases.
“
The way we approach this is we try to meet our clinicians’ pain points,” said Vignesh Shetty, general manager of GE Healthcare’s Edison AI and Platform.
“
Radiology is what a lot of our customers and partners have wanted to work with us [on] so far,” Shetty said in an interview. “For a growing number of these clearances, you will notice that we’ve got use cases in other care pathways as well, including but not limited to oncology, cardiology, and even neurology.”
Meanwhile, Berlin-based Siemens Healthineers had 29 cleared devices, including a feature to help with quick quantification and accurate visualization of calcified coronary lesions from CT scans.
Canon Medical Systems, a subsidiary of the Tokyo-based photography company; Tel Aviv-based Aidoc, which makes computer-aided triage systems; and Amsterdam-based conglomerate Philips also had several cleared AI devices.
5. AI/ML devices will get increasingly complex with algorithms that can “learn” and regulators are looking to adapt
Currently, any major changes to a medical device must be cleared by the FDA, so most algorithms remain static after they’re introduced to the market. Still, the agency is preparing for a future when the software integrated in these devices can adapt.
AI- and machine-learning-enabled devices will become increasingly complex as companies move toward algorithms that “learn” as opposed to algorithms that are “locked and deployed,” wrote IQVIA’s Miller.
“
One thing that is really peculiar for AI devices, the core of the device is an AI algorithm, a piece of software. The piece of software may evolve over time, so there needs to be periodic surveillance of how the software inside of the device is changing,” said Scripps’ Quer.
The FDA has taken some steps to modernize how it regulates these types of devices. It has been working on a digital health pre-certification program that would allow the agency to pre-clear trusted manufacturers to update their software products, though this hasn’t been without hurdles. In a recent report, the FDA acknowledged that it would need Congressional approval to move forward with the program.
The FDA also has discussed a predetermined change control plan, where companies could outline anticipated modifications to software-based devices, allowing them to make changes within those boundaries without making another time-consuming submission to the agency.
Ensuring patients benefit from these devices will depend not only on regulators, but also on developers involving clinicians in the earliest steps of design to ensure software is effective when integrated into their practice.
“
We need to have a deep collaboration between the AI world — the computer scientists or engineers, like myself, who work with the data and the algorithms — and on the other side the clinicians, who are facing the patient and can provide a clinical perspective,” Quer said. “The clinician always needs to be in the loop and provide that clinical input, be the one that judges if this is useful or not.”
Originally published at https://www.medtechdive.com.












