health transformation . institute
knowledge portal & advisory consulting
Joaquim Cardoso MSc
Chief Researcher, Editor & Advisor
December 30, 2022
SOURCE:
Nature Medicine
Carrie Arnold & Paul Webster
23 December 2022
2022 has been a rollercoaster year for biopharma, as it has faced an industry-wide slowdown and late-stage clinical trial failures, as well as breakthroughs and regulatory approvals.
Nature Medicine asks leading researchers to name their top clinical trial for 2023, from cervical and prostate cancer screening to new drugs for Parkinson’s disease and Alzheimer’s disease.
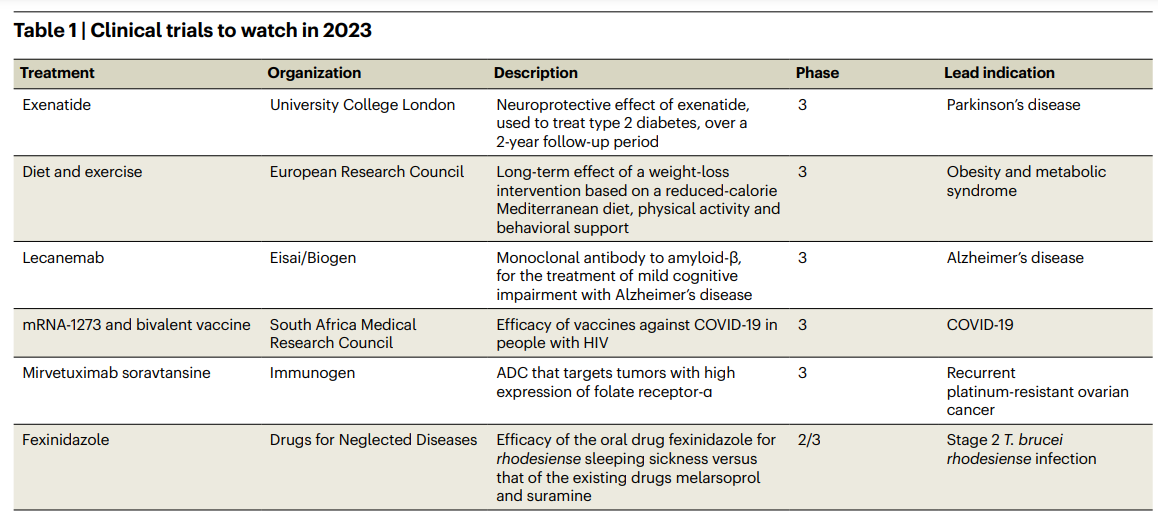
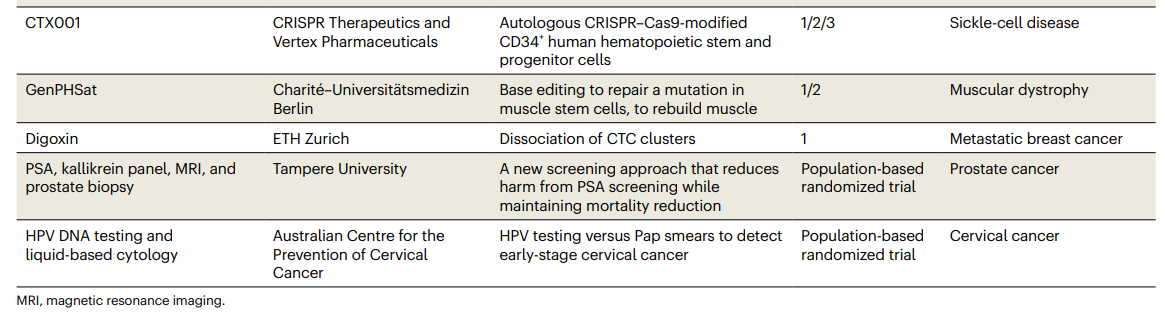
Table 1 Clinical trials to watch in 2023

We asked 11 leading experts for their top clinical trials to watch in the coming year.
- 1.A diabetes drug for Parkinson’s disease
- 2.ADC for ovarian cancer
- 3.CRISPR-Cas9 for muscular dystrophy
- 4.Cervical cancer screening in the vaccinated
- 5.The Mediterranean diet for weight loss
- 6.Safe treatment for sleeping sickness
- 7.Circulating tumor cells
- 8.Lecanemab for Alzheimer’s disease
- 9.COVID-19 vaccination and HIV
- 10.Gene editing for sickle-cell disease
- 11.Reducing harm from prostate cancer screening
1.A diabetes drug for Parkinson’s disease
Roger Albin: For both purely scientific issues and clinical practice issues, the phase 3 trial for exenatide in Parkinson’s disease is a very attractive trial. It has the big advantage of being a repurposed drug that is already widely used in older patients. If there were a positive result, it is something that could be really adopted into clinical practice in a very practical way. The drug had reasonable preclinical data and some promising phase 2 data, and in the Parkinson’s disease world, in which there is not an animal model for really great predictive validity, this is probably about as good as it gets. The community is looking for unequivocal results, whether positive or negative. A clear positive response would be great, but a clear negative response is actually just as important.
Roger Albin is a professor of neurology and co-director of the Movement Disorders Clinic in the Department of Neurology at the University of Michigan Medical School.
2.ADC for ovarian cancer
Robert L. Coleman: The most imminent and important upcoming trial result expected in my field in 2023 is mirvetuximab soravtansine, from ImmunoGen. This received accelerated approval from the US Food and Drug Administration (FDA) on 14 November, based on results of a single-arm trial that enrolled 106 patients with platinum-resistant ovarian cancer whose tumors had high expression of folate receptor-α and who had been treated with up to three prior regimens, at least one of which included bevacizumab (Avastin).
Under accelerated approval, the sponsor can market their drug under the indication agreed to by the FDA — in this case, patients with recurrent, platinum-resistant ovarian cancer. For the drug to move from accelerated approval to regular approval, a confirmatory trial needs to be conducted to confirm the overall safety and efficacy of the agent of interest. In this case, initial results for the confirmatory phase 3 MIRASOL trial are expected in early 2023.
This drug is an antibody-drug conjugate (ADC); these agents are already being used for the treatment of several solid and liquid tumors, but this is the first for ovarian cancer. It will be aligned with a companion diagnostic test that mirrors the expression in tumors needed for clinical trial eligibility. We expect about one-third of patients with recurrent, platinum-resistant ovarian cancer to have high expression of folate receptor-α. The ADC field is expanding rapidly, with trastuzumab deruxtecan approved in April 2022 for use against breast cancer, but it has been a long time since a new cytotoxic agent has been approved for ovarian cancer. Among treatments for gynecological cancers, this is only the second ADC approved so far, after Tisotumab vedotin (in September 2021) for patients with recurrent previously treated cervical cancer. Now we have one in ovarian cancer. Several other ADCs are in development, and successful approval will provide a solid framework for clinical trials evaluating novel combinations in several disease settings.
Robert L. Coleman is chief scientific officer at US Oncology Research.
3.CRISPR-Cas9 for muscular dystrophy
Simone Spuler: Muscle stem cells are the only cells that can regenerate muscle. In patients who have a genetic muscular dystrophy in which muscle wastes for genetic reasons, these stem cells carry mutations, but these mutations can now be corrected with CRISPR-Cas9 and other tools. Correcting muscle stem cells means muscle can be rebuilt, which was not been previously possible whatsoever in these muscular dystrophies.
Muscular dystrophies are a group of about 50 different diseases that lead young people and children to lose their ability to walk, or to breathe, and make them wheelchair-bound within a couple of years. We are working with corrected muscle stem cells able to rebuild muscles and we will test this in a trial called bASKet. In the bASKet trial, there are two major questions. The first is about safety. We would like to see that nothing happens to the patients that makes the disease worse, such as a gene encoding a tumor suppressor being switched on. We do all kinds of preclinical safety tests to preclude that possibility. New proteins will be made by these stem cells, which probably have not been seen by the patient’s immune system, so it could attack this foreign protein. We will inject the cells that are repaired into the patient in an autologous manner, and check a few months later to see if new muscle is built. The second question we are addressing is about clinical improvement. This is something the regulatory agencies asked us to do. We hope to have the first data a few months after we begin treating patients in June and July 2023.
Simone Spuler leads the myology research group and the Outpatient Clinic for Muscle Disorders at the Experimental and Clinical Research Center, a joint institution established by the Max Delbrück Center and the Charité-Universitätsmedizin, in Berlin, Germany.
4.Cervical cancer screening in the vaccinated
Karen Canfell: Prophylactic vaccines against human papilloma virus (HPV), first rolled out 15 years ago, protect women against cervical cancer and are now routinely offered to young girls in most high-income countries. As time moves on, more women who were vaccinated as girls become eligible for cervical cancer screening, and it is important to understand the most effective screening approaches in a vaccinated population. This trial is important, as it is the first large-scale randomized controlled trial internationally that will assess primary HPV screening in a population that is heavily vaccinated against HPV. The findings from the secondary randomization will assess newer approaches for managing HPV-positive women, which will be important for cervical screening programs that are transitioning to primary HPV testing. The COMPASS trial is also assessing new next-generation HPV testing platforms and technologies for triage testing, which are expected to improve the overall performance of HPV testing at a program level.
Karen Canfell is chair of the Cancer Screening and Immunisation Committee of Cancer Council Australia.
5.The Mediterranean diet for weight loss
Jordi Salas Salvadó: No study has ever demonstrated that weight loss and maintenance using an energy-reduced healthy diet and physical activity lowers the risk of cardiovascular disease in people who are overweight or who have obesity. The Look AHEAD trial in the USA, conducted in people with diabetes, has been discontinued owing to lack of efficacy in reducing the risk of cardiovascular events and mortality after approximately 10 years of follow-up, despite achieving significant differences between interventions in long-term weight loss.
We hypothesize that an intensive lifestyle-intervention program aimed at weight loss and based on the traditional Mediterranean diet is a sustainable long-term approach for achieving weight loss in overweight and obese adults, and that the lifestyle changes achieved will have a beneficial effect on cardiovascular morbidity and mortality.
Jordi Salas Salvadó is a Distinguished Professor of Nutrition at Rovira i Virgili University, Spain.
6.Safe treatment for sleeping sickness
Olaf Valverde: In 2023, we will receive the complete results of our clinical trial testing of a breakthrough, all-oral, safe medicine for treating the variant of sleeping sickness caused by Trypanosoma brucei rhodesiense. Also known as human African trypanosomiasis, this neglected parasitic disease transmitted by the bite of the tse tse fly causes severe neuropsychiatric disorders. In contrast to T. brucei gambiense, for which humans are considered the primary reservoir, T. brucei rhodesiense is highly zoonotic, with animals and livestock considered the primary reservoir. It is endemic in eastern and southern Africa, evolves quickly, and can kill in weeks to months if left untreated.
For decades, doctors in endemic countries had to treat sleeping sickness by using melarsoprol, an arsenic derivative so toxic that it killed 5% of patients. Our organization started developing a series of improved drugs, and in 2018 registered fexinidazole, a safe and effective first all-oral drug for the variant of the disease caused by T. brucei gambiense. But for patients with the variant caused by T. brucei rhodesiense, doctors still have to use the dreaded melarsoprol for advanced cases. This clinical trial is assessing the efficacy of fexinidazole for sleeping sickness caused by T. brucei rhodesiense, in comparison with the efficacy of the existing drugs melarsoprol and suramin. Full results will be presented to the European Medicines Agency in 2023, and we expect to get a favorable opinion.
Olaf Valverde is the clinical project leader of the human African trypanosomiasis team of the Drugs for Neglected Diseases initiative.
7.Circulating tumor cells
Nicola Aceto: My lab is interested in metastasis. More than 90% of people with cancer die when metastasis happens. It is a big unsolved problem. We recently found that metastasis is driven mostly by clusters of circulating tumor cells (CTCs), which are multicellular aggregates of tumor cells that depart from the existing tumor, circulate in the bloodstream, and then metastasize. This finding challenged the prevailing dogma in the metastasis field, as until a few years ago, people thought metastasis happened one cell at a time. Thanks to new technologies, we could finally investigate blood samples from patients and in animal models, which allowed us to identify CTC clusters.
We have also found that there are drugs, such as digoxin, that have the ability to dissociate these cells and dissolve the clusters, which shuts down metastasis in preclinical models. We have now set up a small phase 1 trial as a proof of mechanism. We screen the blood of patients with advanced metastatic breast cancer, and when we find CTC clusters, we give the patients the drug for 3 weeks, during which time we measure the abundance and features of the clusters. Digoxin is a well-known drug used to treat heart conditions, but it has this beautiful side effect. Should the trial be successful, we envision the generation of improved cluster-dissociating molecules, able to achieve full cluster dissolution and specifically designed to treat cancer. This is the next ambitious goal: enabling a novel cancer-treatment modality that is aimed at blocking the spread of cancer.
Nicola Aceto is an associate professor of molecular oncology at ETH Zurich.
8.Lecanemab for Alzheimer’s disease
Allan Levey: In 2023, I expect to see more peer-reviewed publications and data on lecanemab, an investigational monoclonal antibody to amyloid-β protofibrils, for the treatment of mild cognitive impairment with Alzheimer’s disease. The developer, Eisai, announced positive topline results from their large global phase 3 confirmatory Clarity AD clinical trial of lecanemab in late September. We saw extensive data on lecanemab at the Clinical Trials on Alzheimer’s Congress in late November and early December 2022 and a landmark publication in the New England Journal of Medicine was published on 29 November 2022.
Much data have been made available for scrutiny and independent, secondary analyses with the publication. Eisai is expected to file an application with the FDA for traditional approval in the USA and marketing-authorization applications in Japan and Europe by the end of March 2023. This is a pivotal phase 3 trial that most experts consider a huge game-changer for this field. In Alzheimer’s disease, there are no disease-modifying treatments that are clearly proven (aducanumab has been approved, despite uncertain clinical efficacy). Until recently, evidence for disease modification has been lacking, despite an industry-wide focus on amyloid-based therapies for many years.
With this new lecanemab study, the results of the phase 3 study show a significant reduction in clinical progression, confirming the results of an earlier phase 2 study. All primary and secondary endpoints, including dementia severity, cognition and functional abilities, were met. The second issue is that safety has been a huge concern with previous treatments given accelerated approval. The results for lecanemab indicate that its safety is much better, although there were adverse events. These are the reasons it is a game changer. Additional important insights will be gleaned about the magnitude and duration of benefits, and a more palatable and scalable form of subcutaneous dosing when more data and analysis are published in 2023.
Allan Levey is a professor and chair of the Department of Neurology at Emory University’s School of Medicine, and director of the Emory University Goizueta Alzheimer’s Disease Research Center.
9.COVID-19 vaccination and HIV
Glenda Gray: In December 2021, we began a trial to enroll almost 14,500 participants in more than 50 research clinics in eight sub-Saharan African countries. The Ubuntu multicenter phase 3 clinical trial will assess the efficacy of the mRNA-1273 (Moderna) vaccine against COVID-19 in adults infected with human immunodeficiency virus (HIV) or with other comorbidities that increase the risk of severe COVID-19. This trial will include a smaller number of HIV-negative people.
There is an urgent need to characterize infection and viral clearance in people who are immunocompromised, which will be assessed in our study. The results, which we expect in 2023, should indicate how many doses of vaccine are needed for adults living with HIV, as well as in adults with other health conditions that may put them at risk for severe COVID-19. We are also expecting data on whether people who have been infected with the coronavirus SARS-CoV-2, and therefore probably have some immunity, need as many vaccine doses as those without prior infection. We also want to know if the original Moderna vaccine is inferior to the new bivalent one, which includes the spike protein from a SARS-CoV-2 variant of concern. This direct comparison of mRNA-1273 against the bivalent vaccine should give us insight into the utility of variant-specific vaccines. We hope that when these results are published next year, they will help to refine an optimal vaccine strategy and the best regimen for HIV-infected people.
Glenda Gray is president and CEO of the South Africa Medical Research Council.
10.Gene editing for sickle-cell disease
Luigi Naldini: We are all waiting for the first long-term data from gene-editing strategies in sickle-cell disease and thalassemia. There have been preliminary reports of efficient editing. The key question is whether these gene grafts remain stable. We have seen very safe stable long-term grafts of stem cells treated with lentiviral vectors, and prolonged safety, but will this be the same for gene-editing tools?
We may soon be seeing the interim results in 2023 of a multi-center sickle-cell disease trial of gene editing sponsored by CRISPR Therapeutics and Vertex Pharmaceuticals. This is a single-arm, open-label, multi-site, single-dose phase 1/2/3 study in people with severe sickle-cell disease. The study is evaluating the safety and efficacy of autologous CRISPR-Cas9-modified CD34+human hematopoietic stem and progenitor cells. Participants receive a single infusion of these cells through a central venous catheter. The top outcome of interest would be participants who have not experienced any severe vaso-occlusive crisis for at least 12 consecutive months. It will be crucial to verify the long-term stability and polyclonal composition of the graft without the emergence of adverse events. Beyond this, what we are looking forward to seeing is the first clinical testing of what we call ‘writing back’ genes; that is, correcting genetic mutations by introducing editing of longer sequences, which has not been clinically achieved yet. We and others are actively working closely on that.
Luigi Naldini is a professor of cell and tissue biology and of gene and cell therapy at the San Raffaele University School of Medicine, and scientific director of the San Raffaele Telethon Institute for Gene Therapy, Milan, Italy.
11.Reducing harm from prostate cancer screening
Anssi Auvinen: The evidence surrounding testing for the marker PSA (prostate-specific antigen) is full of conflict, as the test may detect prostate cancer but at the expense of treating cancers with little threat to health. We aim to detect only clinically relevant, aggressive prostate cancer while minimizing the diagnosis of clinically unimportant, low-risk cancers that would constitute over-diagnosis (meaning that they would not progress even if left undetected and untreated). A previous trial showed benefits that were comparable to those of other cancer screening programs, but we wanted to put more effort into harnessing recent developments to reduce harm, including over-diagnosis and unnecessary biopsies.
Anssi Auvinen is a professor of health sciences at Tampere University, Finland.
Cite this article
Arnold, C., Webster, P. 11 clinical trials that will shape medicine in 2023. Nat Med 28, 2444–2448 (2022). https://doi.org/10.1038/s41591-022-02132-3
Originally published at https://www.nature.com on December 23, 2022.
Names mentioned & affiliations
Roger Albin is a professor of neurology and co-director of the Movement Disorders Clinic in the Department of Neurology at the University of Michigan Medical School.
Robert L. Coleman is chief scientific officer at US Oncology Research.
Simone Spuler leads the myology research group and the Outpatient Clinic for Muscle Disorders at the Experimental and Clinical Research Center, a joint institution established by the Max Delbrück Center and the Charité-Universitätsmedizin, in Berlin, Germany.
Karen Canfell is chair of the Cancer Screening and Immunisation Committee of Cancer Council Australia.
Jordi Salas Salvadó is a Distinguished Professor of Nutrition at Rovira i Virgili University, Spain.
Olaf Valverde is the clinical project leader of the human African trypanosomiasis team of the Drugs for Neglected Diseases initiative.
Nicola Aceto is an associate professor of molecular oncology at ETH Zurich.
Allan Levey is a professor and chair of the Department of Neurology at Emory University’s School of Medicine, and director of the Emory University Goizueta Alzheimer’s Disease Research Center.
Glenda Gray is president and CEO of the South Africa Medical Research Council.
Luigi Naldini is a professor of cell and tissue biology and of gene and cell therapy at the San Raffaele University School of Medicine, and scientific director of the San Raffaele Telethon Institute for Gene Therapy, Milan, Italy.
Anssi Auvinen is a professor of health sciences at Tampere University, Finland.