Health Transformation Institute (HTI)
Joaquim Cardoso MSc*
Founder, and Chief Researcher & Editor
December 1, 2022
MSc* from London Business School
MIT Sloan Masters Program
Senior Advisor for Health Transformation & Digital Health

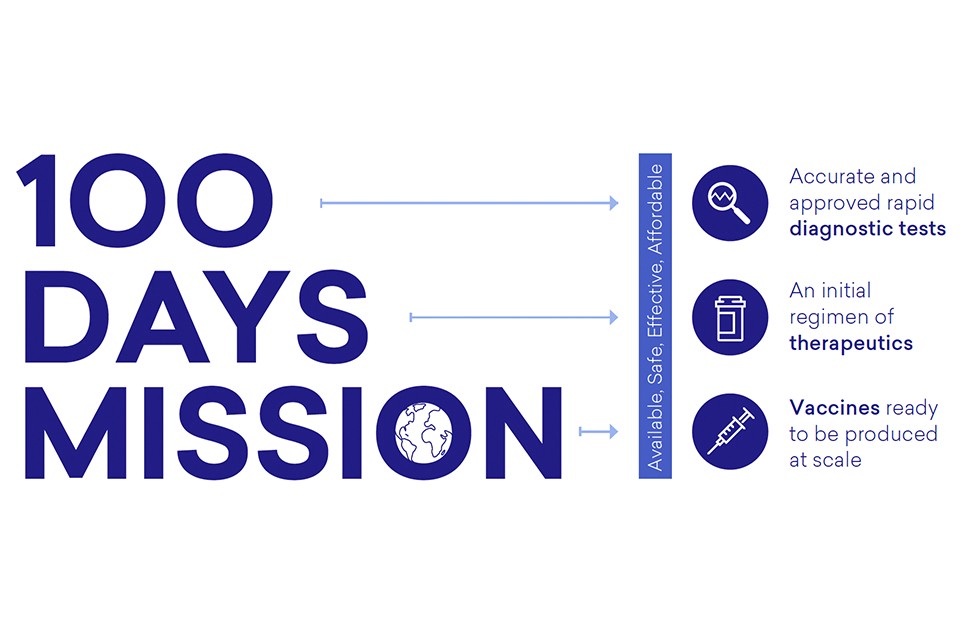
Epidemic coalition sets out plan to develop future vaccines in 100 days
Financial Times
November 29, 2022
A practical blueprint to develop a vaccine against a new virus within 100 days — less than a third of the time taken to produce Covid-19 vaccines in 2020 — will be released on Wednesday by an international organisation charged with protecting against future pandemics.
The Coalition for Epidemic Preparedness Innovations had produced a detailed plan which shows that “the 100-day mission is not just a slogan or a tool for fundraising” but a practical proposition, said Cepi chief executive Richard Hatchett.
It was prepared through in-depth research and consultation with international health bodies, vaccine companies, scientists and regulators.
Cepi, a global partnership between governments, charities and industry set up in 2017, aims to raise $3.5bn for a five-year programme that would enable the world to supercharge the development and manufacturing of vaccines against any emerging virus with pandemic potential.
Cepi, a global partnership between governments, charities and industry set up in 2017, aims to raise $3.5bn for a five-year programme …
… that would enable the world to supercharge the development and manufacturing of vaccines against any emerging virus with pandemic potential.
“We have pledges of $1.6bn-$1.7bn towards the $3.5bn and we have line of sight on maybe another $800mn to $1bn in potential commitments that have not yet been announced publicly,” said Hatchett.
The target is to have a vaccine that has gone through initial clinical trials and been approved for emergency use within 100 days of a virus being recognised as a pandemic threat by the scientific community and its genetic sequence released.
According to Cepi, the fastest comparable figure for Covid was 326 days between Chinese scientists publishing the Sars-Cov-2 viral genome in January 2020 and the UK Medicines and Healthcare products Regulatory Agency approving the BioNTech/Pfizer vaccine the following December.

As with Covid, initial development work might have to begin well before a disease outbreak is officially recognised as an international health emergency or pandemic, Hatchett said.
“Once a new pathogen has demonstrated some degree of human to human transmission and some evidence of severity, you have to make a value judgment and do some things at risk because you can’t regain lost time,” he said.
“Those first investments are relatively small, several million dollars, and don’t commit you to spending hundreds of millions on day one.”
Cepi envisages five main areas of innovation that will be required for the 100-days mission to succeed:
- creating libraries of prototype vaccines for the virus families most likely to cause a pandemic;
- preparing clinical trial networks to be mobilised very rapidly when needed;
- finding biological markers to give an early indication of immune response to a vaccine;
- establishing biomanufacturing facilities around the world that can switch quickly to making the pandemic product; and
- strengthening global disease surveillance.
Dame Kate Bingham, who led the UK’s Vaccine Taskforce in 2020, said the 100-days mission was “do-able but relies on very close international collaboration and leadership to prepare in advance for Disease X”.
… the 100-days mission is doable, but relies on very close international collaboration and leadership to prepare in advance for Disease X”.
“Comprehensive global surveillance will be essential to identify potential risks rapidly, together with continued investment in diagnostics, manufacturing and clinical development capability around the world,” she added.
“Comprehensive global surveillance will be essential to identify potential risks rapidly, …
… together with continued investment in diagnostics, manufacturing and clinical development capability around the world,” …

Last week the World Health Organization launched a process to update its list of “priority pathogens” most likely to cause a pandemic.
About a dozen families of viruses are prime suspects.
“I think it would be extremely unlikely that we encounter a completely novel family of viruses — and the first pathogen to emerge from it has pandemic potential,” said Hatchett.
Although bacteria have caused terrible epidemics in the past, including the plague, they are regarded as less of a pandemic threat than viruses in the 21st century.
Although bacteria have caused terrible epidemics in the past, including the plague, they are regarded as less of a pandemic threat than viruses in the 21st century.

It is also essential to build up experience with vaccine technology platforms, according to the Cepi plan.
Although mRNA turned out to be the fastest route to effective Covid vaccines, “there may be a reason why other approaches are better for different threats,” Hatchett said, referring to the BioNTech/Pfizer and Moderna jabs.
Although mRNA turned out to be the fastest route to effective Covid vaccines, “there may be a reason why other approaches are better for different threats,
Originally published at https://www.ft.com on November 29, 2022.
Names mentioned
The Coalition for Epidemic Preparedness Innovations
Richard Hatchett
Dame Kate Bingham, who led the UK’s Vaccine Taskforce in 2020